Brand Name
Ga-68 PSMA-11
Generic Name
Ga-68 Gozetotide
View Brand Information FDA approval date: December 09, 2020
Classification: Radioactive Diagnostic Agent
Form: Injection
What is Ga-68 PSMA-11 (Ga-68 Gozetotide)?
Gallium Ga 68 Gozetotide Injection is indicated for positron emission tomography of prostate-specific membrane antigen positive lesions in men with prostate cancer: with suspected metastasis who are candidates for initial definitive therapy. with suspected recurrence based on elevated serum prostate-specific antigen level. Gallium Ga 68 Gozetotide Injection is a radioactive diagnostic agent indicated for positron emission tomography of prostate-specific membrane antigen positive lesions in men with prostate cancer: with suspected metastasis who are candidates for initial definitive therapy. with suspected recurrence based on elevated serum prostate-specific antigen level.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Gallium Ga-68 PSMA-11 (GALLIUM GA-68 GOZETOTIDE)
1INDICATIONS AND USAGE
Gallium Ga 68 Gozetotide Injection is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer:
- with suspected metastasis who are candidates for initial definitive therapy.
- with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level.
2DOSAGE FORMS AND STRENGTHS
Injection: supplied as a clear, colorless solution in a multiple-dose vial containing 18.5 MBq/mL to 185 MBq/mL (0.5 mCi/mL to 5 mCi/mL) of gallium Ga 68 gozetotide in approximately 12 mL at calibration time.
3CONTRAINDICATIONS
None
4OVERDOSAGE
In the event of an overdose of Gallium Ga 68 Gozetotide Injection, reduce the radiation absorbed dose to the patient where possible by increasing the elimination of the drug from the body using hydration and frequent bladder voiding. A diuretic might also be considered. If possible, an estimate of the radiation effective dose given to the patient should be made.
5CLINICAL STUDIES
The safety and efficacy of Gallium Ga 68 Gozetotide Injection were established in two prospective, open-label studies, PSMA-PreRP (NCT03368547 and NCT02919111) and PSMA-BCR (NCT02940262 and NCT02918357) in men with prostate cancer.
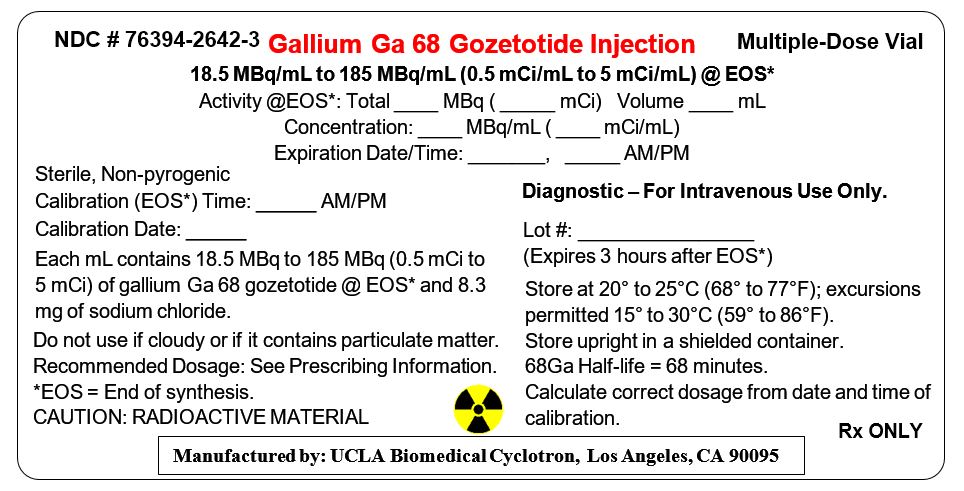
6PRINCIPAL DISPLAY PANEL - 12 mL Vial Label
NDC # 76394-2642-3
18.5 MBq/mL to 185 MBq/mL (0.5 mCi/mL to 5 mCi/mL) @ EOS*
Sterile, Non-pyrogenic
Each mL contains 18.5 MBq to 185 MBq (0.5 mCi to
Do not use if cloudy or if it contains particulate matter.
Diagnostic – For Intravenous Use Only.
Lot #: ________________
Store at 20° to 25°C (68° to 77°F);
Rx ONLY
Manufactured by: UCLA Biomedical Cyclotron, Los Angeles, CA 90095