Brand Name
Sutent
Generic Name
Sunitinib
View Brand Information FDA approval date: January 26, 2006
Classification: Kinase Inhibitor
Form: Capsule
What is Sutent (Sunitinib)?
Sunitinib malate capsules are a kinase inhibitor indicated for: treatment of adult patients with gastrointestinal stromal tumor after disease progression on or intolerance to imatinib mesylate.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
SUTENT (Sunitinib malate)
WARNING: HEPATOTOXICITY
Hepatotoxicity may be severe, and in some cases, fatal. Monitor hepatic function and interrupt, dose reduce, or discontinue SUTENT as recommended
1DOSAGE FORMS AND STRENGTHS
Capsules, hard gelatin:
- 12.5 mg sunitinib: orange cap and orange body, printed with white ink “Pfizer” on the cap and “STN 12.5 mg” on the body.
- 25 mg sunitinib: caramel cap and orange body, printed with white ink “Pfizer” on the cap and “STN 25 mg” on the body.
- 37.5 mg sunitinib: yellow cap and yellow body, printed with black ink “Pfizer” on the cap and “STN 37.5 mg” on the body.
- 50 mg sunitinib: caramel top and caramel body, printed with white ink “Pfizer” on the cap and “STN 50 mg” on the body.
2CONTRAINDICATIONS
None.
3ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling.
- Hepatotoxicity
- Cardiovascular Events
- QT Interval Prolongation and Torsade de Pointes
- Hypertension
- Hemorrhagic Events
- Tumor Lysis Syndrome
- Thrombotic Microangiopathy
- Proteinuria
- Dermatologic Toxicities
- Reversible Posterior Leukoencephalopathy Syndrome
- Thyroid Dysfunction
- Hypoglycemia
- Osteonecrosis of the Jaw
- Impaired Wound Healing
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population described in the Warnings and Precautions reflect exposure to SUTENT in 7527 patients with GIST, RCC (advanced and adjuvant), or pNET. In this pooled safety population, the most common adverse reactions (≥25%) were fatigue/asthenia, diarrhea, mucositis/stomatitis, nausea, decreased appetite/anorexia, vomiting, abdominal pain, hand-foot syndrome, hypertension, bleeding events, dysgeusia/altered taste, dyspepsia, and thrombocytopenia.
3.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of SUTENT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and lymphatic system disorders: hemorrhage associated with thrombocytopenia
including some fatalities . - Gastrointestinal disorders: esophagitis.
- Hepatobiliary disorders: cholecystitis, particularly acalculous cholecystitis.
- Immune system disorders: hypersensitivity reactions, including angioedema.
- Infections and infestations: serious infection (with or without neutropenia)
. The infections most commonly observed with SUTENT include respiratory, urinary tract, skin infections, and sepsis/septic shock. - Musculoskeletal and connective tissue disorders: fistula formation, sometimes associated with tumor necrosis and/or regression
; myopathy and/or rhabdomyolysis with or without acute renal failure . - Renal and urinary disorders: renal impairment and/or failure
. - Respiratory disorders: pulmonary embolism
, pleural effusion . - Skin and subcutaneous tissue disorders: pyoderma gangrenosum, including positive de-challenges.
- Vascular disorders: arterial (including aortic) aneurysms, dissections
, and rupture ; arterial thromboembolic events . The most frequent events included cerebrovascular accident, transient ischemic attack, and cerebral infarction. - General disorders and administration site conditions: impaired wound healing.
4OVERDOSAGE
Treatment of overdose with SUTENT should consist of general supportive measures. There is no specific antidote for overdosage with SUTENT. If indicated, elimination of unabsorbed drug should be achieved by emesis or gastric lavage. Cases of accidental overdose have been reported; these cases were associated with adverse reactions consistent with the known safety profile of SUTENT, or without adverse reactions. In nonclinical studies, mortality was observed following as few as 5 daily doses of 500 mg/kg (3000 mg/m
5DESCRIPTION
Sunitinib is a kinase inhibitor present in SUTENT capsules as the malate salt. Sunitinib malate is described chemically as (2
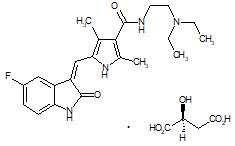
Sunitinib malate is a yellow to orange powder with a pKa of 8.95. The solubility of sunitinib malate in aqueous media over the range pH 1.2 to pH 6.8 is in excess of 25 mg/mL. The log of the distribution coefficient (octanol/water) at pH 7 is 5.2.
SUTENT (sunitinib malate) capsules are supplied as printed hard shell capsules containing 12.5 mg, 25 mg, 37.5 mg or 50 mg of sunitinib (equivalent to 16.7 mg, 33.4 mg, 50.1 mg, or 66.8 mg of sunitinib malate, respectively). The capsules contain the following inactive ingredients: croscarmellose sodium, magnesium stearate, mannitol, and povidone (K-25). The orange gelatin capsule shells contain titanium dioxide and red iron oxide; the caramel gelatin capsule shells contain titanium dioxide, red iron oxide, yellow iron oxide, and black iron oxide; and the yellow gelatin capsule shells contain titanium dioxide and yellow iron oxide. The white printing ink contains shellac, propylene glycol, sodium hydroxide, povidone, and titanium dioxide and the black printing ink contains shellac, propylene glycol, potassium hydroxide, and black iron oxide.
6HOW SUPPLIED/STORAGE AND HANDLING
SUTENT 12.5 mg capsules are supplied as hard gelatin capsule with orange cap and orange body, printed with white ink “Pfizer” on the cap, “STN 12.5 mg” on the body:
SUTENT 25 mg capsules are supplied as hard gelatin capsule with caramel cap and orange body, printed with white ink “Pfizer” on the cap, “STN 25 mg” on the body:
SUTENT 37.5 mg capsules are supplied as hard gelatin capsule with yellow cap and yellow body, printed with black ink “Pfizer” on the cap, “STN 37.5 mg” on the body:
SUTENT 50 mg capsules are supplied as hard gelatin capsule with caramel cap and caramel body, printed with white ink “Pfizer” on the cap, “STN 50 mg” on the body:
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
8PRINCIPAL DISPLAY PANEL - 12.5 mg Capsule Bottle Label
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0069-0550-38
Pfizer
Sutent
12.5 mg*
Capsules
28 Capsules

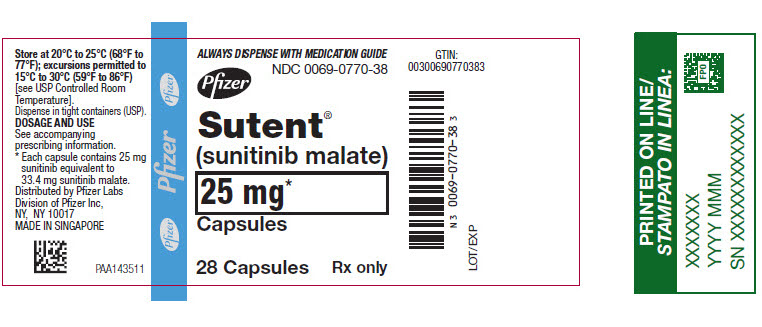
9PRINCIPAL DISPLAY PANEL - 25 mg Capsule Bottle Label
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0069-0770-38
Pfizer
Sutent
25 mg*
Capsules
28 Capsules
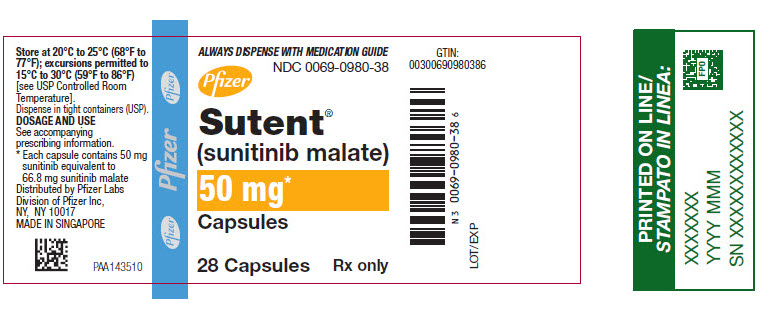
10PRINCIPAL DISPLAY PANEL - 50 mg Capsule Bottle Label
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0069-0980-38
Pfizer
Sutent
50 mg*
Capsules
28 Capsules
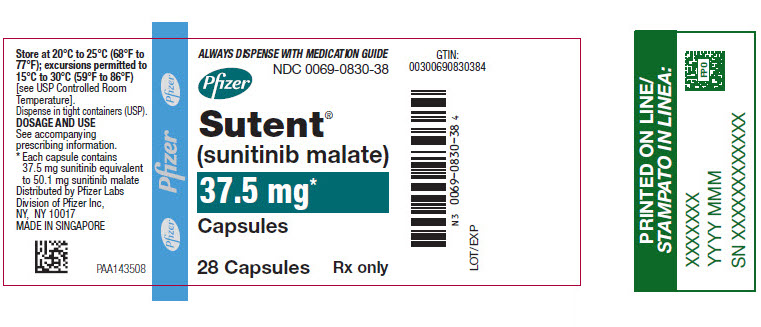
11PRINCIPAL DISPLAY PANEL - 37.5 mg Capsule Bottle Label
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0069-0830-38
Pfizer
Sutent
37.5 mg*
Capsules
28 Capsules