Brand Name
Altoprev
Generic Name
Lovastatin
View Brand Information FDA approval date: December 17, 2001
Classification: HMG-CoA Reductase Inhibitor
Form: Tablet
What is Altoprev (Lovastatin)?
Therapy with lovastatin tablets USP should be a component of multiple risk factor intervention in those individuals with dyslipidemia at risk for atherosclerotic vascular disease. Lovastatin tablets USP should be used in addition to a diet restricted in saturated fat and cholesterol as part of a treatment strategy to lower total-C and LDL-C to target levels when the response to diet and other nonpharmacological measures alone has been inadequate to reduce risk. Primary Prevention of Coronary Heart Disease In individuals without symptomatic cardiovascular disease, average to moderately elevated total-C and LDL-C and below average HDL-C, lovastatin tablets USP are indicated to reduce the risk of: -Myocardial infarction -Unstable angina -Coronary revascularization procedures . Coronary Heart Disease Lovastatin tablets USP are indicated to slow the progression of coronary atherosclerosis in patients with coronary heart disease as part of a treatment strategy to lower total-C and LDL-C to target levels. Hypercholesterolemia Therapy with lipid-altering agents should be a component of multiple risk factor intervention in those individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia. Lovastatin tablets USP are indicated as an adjunct to diet for the reduction of elevated total-C and LDL-C levels in patients with primary hypercholesterolemia , when the response to diet restricted in saturated fat and cholesterol and to other nonpharmacological measures alone has been inadequate. 2 Classification of Hyperlipoproteinemias IDL = intermediate-density lipoprotein. Type Lipoproteins elevated Lipid Elevations major minor I chylomicrons TG ↑→C IIa LDL C — IIb LDL,VLDL C TG III IDL C/TG — IV VLDL TG ↑→C V chylomicrons,VLDL TG ↑→C Adolescent Patients with Heterozygous Familial Hypercholesterolemia Lovastatin tablets USP are indicated as an adjunct to diet to reduce total-C, LDL-C and apolipoprotein B levels in adolescent boys and girls who are at least one year post-menarche, 10 to 17 years of age, with heFH if after an adequate trial of diet therapy the following findings are present: 1. LDL-C remains >189 mg/dL or 2. LDL-C remains >160 mg/dL and: there is a positive family history of premature cardiovascular disease or two or more other CVD risk factors are present in the adolescent patient General Recommendations Prior to initiating therapy with lovastatin, secondary causes for hypercholesterolemia should be excluded, and a lipid profile performed to measure total-C, HDL-C, and TG. For patients with TG less than 400 mg / dL.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Altoprev (lovastatin)
1INDICATIONS AND USAGE
Altoprev is indicated:
- To reduce the risk of myocardial infarction, unstable angina, and coronary revascularization procedures in adults at high risk for coronary heart disease.
- As an adjunct to diet to reduce low-density lipoprotein cholesterol (LDL-C) and slow the progression of coronary atherosclerosis in adults with coronary heart disease.
- As an adjunct to diet to reduce LDL-C in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (HeFH).
2DOSAGE FORMS AND STRENGTHS
Altoprev extended-release tablets are available as follows:
3CONTRAINDICATIONS
Altoprev is contraindicated in the following conditions:
- Concomitant administration of strong CYP3A inhibitors and erythromycin
- Acute liver failure or decompensated cirrhosis
- Hypersensitivity to lovastatin or any excipients in Altoprev. Hypersensitivity reactions, including anaphylaxis, angioedema and Stevens-Johnson syndrome, have been reported
4ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Myopathy and Rhabdomyolysis
- Immune-Mediated Necrotizing Myopathy
- Hepatic Dysfunction
- Increases in HbA1c and Fasting Serum Glucose Levels
4.1Clinical Trial Adverse Reactions
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials 467 patients were treated with Altoprev with mean exposure of approximately 11.6 weeks. The mean age of the population was 56 years, 43% of the population were female, 86% were White, 6% were Black or African American, 1% were Asian, and 7% were other races
Elevations in Liver Enzyme Tests
In the AFCAPS/TexCAPS study, 6,605 patients were treated with lovastatin immediate-release (n=3,304) or placebo (n=3,301). Patients with consecutive elevations of either alanine aminotransferase (ALT) or aspartate aminotransferase (AST) (>3 times ULN), over a median of 5.1 years of follow-up, was not significantly different between the lovastatin immediate-release and placebo groups. Elevated transaminases resulted in discontinuation of 6 (0.2%) patients from therapy in the lovastatin immediate-release group and 4 (0.1%) in the placebo group.
In the EXCEL study, the incidence of persistent increases in serum transaminases over 48 weeks was 0.1% for placebo, 0.1% at 20 mg daily, 0.9% at 40 mg daily, and 1.5% at 80 mg daily in patients treated with lovastatin immediate-release (not an approved dose of Altoprev)
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Altoprev. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skeletal: muscle cramps, myopathy, rhabdomyolysis. There have been rare reports of immune-mediated necrotizing myopathy associated with statin use.
Neurological: dysfunction of certain cranial nerves (including alteration of taste, impairment of extra-ocular movement, facial paresis), tremor, vertigo, paresthesia, peripheral neuropathy, peripheral nerve palsy, psychic disturbances, anxiety, insomnia, depression.
There have been rare post marketing reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with statin use. These cognitive issues have been reported for all statins. The reports are generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks). There have been rare reports of new-onset or exacerbation of myasthenia gravis, including ocular myasthenia, and reports of recurrence when the same or a different statin was administered.
Hypersensitivity Reactions: An apparent hypersensitivity syndrome has been reported rarely which has included one or more of the following features: anaphylaxis, angioedema, lupus erythematous-like syndrome, polymyalgia rheumatica, dermatomyositis, vasculitis, purpura, thrombocytopenia, leukopenia, hemolytic anemia, positive ANA, ESR increase, eosinophilia, arthritis, urticaria, photosensitivity, fever, chills, flushing, malaise, dyspnea, toxic epidermal necrolysis, erythema multiforme, including Stevens-Johnson syndrome.
Gastrointestinal: pancreatitis, hepatitis, including chronic active hepatitis, cholestatic jaundice, fatty change in liver; and rarely, cirrhosis, fulminant hepatic necrosis, and hepatoma; anorexia, vomiting, fatal and non-fatal hepatic failure.
Skin: alopecia, pruritus, lichen planus. A variety of skin changes (e.g., nodules, discoloration, dryness of skin/mucous membranes, changes to hair/nails) have been reported.
Reproductive: gynecomastia, loss of libido, erectile dysfunction.
Eye: progression of cataracts (lens opacities), ophthalmoplegia.
Laboratory Abnormalities: alkaline phosphatase, g-glutamyl transpeptidase, and bilirubin; thyroid function abnormalities.
Respiratory: interstitial lung disease.
5OVERDOSAGE
No specific antidotes for Altoprev are known. In the event of an overdose of Altoprev, consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
6DESCRIPTION
Altoprev (lovastatin extended-release) tablets are an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase (statin) isolated from a strain of
Lovastatin is [1
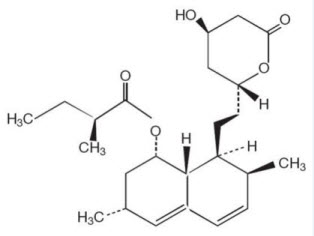
Lovastatin is a white, nonhygroscopic crystalline powder that is insoluble in water and sparingly soluble in ethanol, methanol, and acetonitrile.
Altoprev extended-release tablets for oral use contain 20 mg, 40 mg, or 60 mg of lovastatin. In addition, each tablet contains the following inactive ingredients: acetyltributyl citrate; butylated hydroxy anisole; candelilla wax; cellulose acetate; confectioner’s sugar (contains corn starch); F D & C yellow # 6; glyceryl monostearate; hypromellose; hypromellose phthalate; lactose; methacrylic acid copolymer, type B; polyethylene glycols (PEG 400, PEG 8000); polyethylene oxides; polysorbate 80; propylene glycol; silicon dioxide; sodium chloride; sodium lauryl sulfate; synthetic black iron oxide; red iron oxide; talc; titanium dioxide and triacetin.
7CLINICAL STUDIES
Primary Hyperlipidemia in Adults
Altoprev has been shown to reduce Total-C, LDL-C, and TG and increase HDL-C in patients with hyperlipidemia. Near maximal response was observed after four weeks of treatment and the response was maintained with continuation of therapy for up to 6 months.
In a 12-week, multicenter, placebo-controlled, double-blind, trial in adult males and females 21 to 70 years of age with primary hyperlipidemia, once daily administration of Altoprev 20 to 60 mg in the evening was compared to placebo. Altoprev produced dose related reductions in LDL-C and Total-C. The lipid changes with Altoprev treatment in this trial, from baseline to endpoint, are displayed in
The range of LDL-C responses is represented graphically in the following figure (
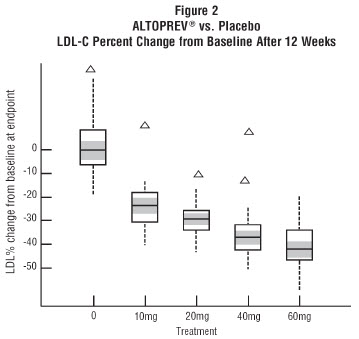
The distribution of LDL-C responses is represented graphically by the boxplots in Figure 1. The bottom line of the box represents the 25th percentile and the top line, the 75th percentile. The horizontal line in the box represents the median and the gray area is the 95% confidence interval for the median. The range of responses is depicted by the tails and outliers.
Expanded Clinical Evaluation of Lovastatin Immediate-Release (EXCEL) Study
Lovastatin immediate-release was compared to placebo in 8,245 patients with hyperlipidemia [Total-C 240 to 300mg/dL, LDL-C >160 mg/dL] in the randomized, double-blind, parallel, 48-week EXCEL trial. All changes in the lipid measurements (see
Heterozygous Familial Hypercholesterolemia in Adults
Lovastatin immediate-release has been shown to be effective in reducing Total-C and LDL-C in heterozygous familial and non-familial forms of primary hypercholesterolemia. A response was seen within 2 weeks, and the maximum response occurred within 4-6 weeks. The response was maintained during continuation of therapy.
Prevention of Coronary Heart Disease
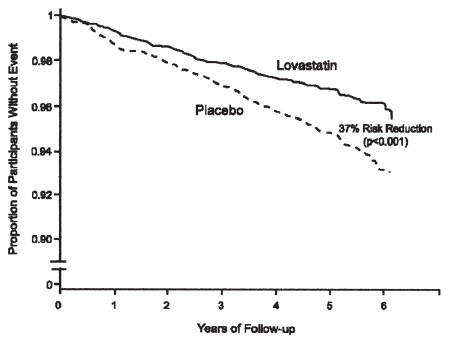
The Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS), a double-blind, randomized, placebo-controlled, primary prevention trial, demonstrated that treatment with lovastatin immediate-release decreased the rate of acute major coronary events (composite endpoint of myocardial infarction, unstable angina, and sudden cardiac death) compared with placebo during a median of 5.1 years of follow-up. Participants were males (ages 45 to 73) and females (ages 55 to 73) without symptomatic cardiovascular disease with average to moderately elevated Total-C and LDL-C, below average HDL-C, and who were at high risk based on elevated Total-C/HDL-C. In addition to age, 63% of the participants had at least one other risk factor (baseline HDL-C <35 mg/dL, hypertension, family history, smoking and diabetes).
AFCAPS/TexCAPS enrolled 6,605 participants (5,608 males, 997 females) based on the following lipid entry criteria: Total-C range of 180 to 264 mg/dL, LDL-C range of 130 to 190 mg/dL, HDL-C of ≤45 mg/dL for males and ≤47 mg/dL for females, and TG of ≤400 mg/dL. Participants were treated with standard care, including diet, and either lovastatin immediate-release 20 mg to 40 mg daily (n= 3,304) or placebo (n= 3,301). Approximately 50% of the participants treated with lovastatin immediate-release were titrated to 40 mg daily when their LDL-C remained >110 mg/dL at the 20-mg starting dose.
Lovastatin immediate-release reduced the risk of a first acute major coronary event, the primary efficacy endpoint, by 37% (lovastatin immediate-release 3.5%, placebo 5.5%; p<0.001;
Atherosclerosis
In the Canadian Coronary Atherosclerosis Intervention Trial (CCAIT), the effect of therapy with lovastatin immediate-release on coronary atherosclerosis was assessed by coronary angiography in hyperlipidemic patients. In this randomized, double-blind, controlled clinical trial, patients were treated with conventional measures (usually diet and 325 mg of aspirin every other day) and either lovastatin immediate-release 20 mg to 80 mg daily or placebo (the 80 mg dose is not approved for Altoprev
8HOW SUPPLIED/STORAGE AND HANDLING
Altoprev extended-release tablets are supplied as follows:
Storage
Store at 20-25°C (68-77°F) - Excursions Permitted to 15°C -30°C (59°F -86°F) [See USP Controlled Room Temperature]. Avoid excessive heat and humidity.
9PATIENT COUNSELING INFORMATION
Myopathy and Rhabdomyolysis
Advise patients that Altoprev may cause myopathy and rhabdomyolysis. Inform patients that the risk is also increased when taking certain types of medication or consuming grapefruit juice and they should discuss all medication, both prescription and over the counter, with their healthcare provider. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever
Hepatic Dysfunction
Inform patients that Altoprev may cause liver enzyme elevations and possibly liver failure. Advise patients to promptly report fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice
Increases in HbA1c and Fasting Serum Glucose Levels
Inform patients that increases in HbA1c and fasting serum glucose levels may occur with Altoprev. Encourage patients to optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices
Pregnancy
Advise pregnant patients and patients who can become pregnant of the potential risk to a fetus. Advise patients to inform their healthcare provider of a known or suspected pregnancy to discuss if Altoprev should be discontinued
Lactation
Advise patients that breastfeeding is not recommended during treatment with Altoprev
Missed Dose
Instruct patients to take Altoprev only as prescribed. If a dose is missed, it should be taken as soon as possible. Advise patients not to double their next dose.
10PRINCIPAL DISPLAY PANEL - 20 mg BOTTLE

NDC 70515-628-30
Altoprev
(lovastatin)
extended-release tablets
(lovastatin)
extended-release tablets
20 mg
Rx only
COVIS
Each tablet contains lovastatin 20 mg.
Read package insert for prescribing
KEEP OUT OF THE REACH OF CHILDREN.
Dispense in tight, light-resistant container
Store at 20°-25°C (68°-77°F). Excursions
Avoid excessive heat and humidity.
Manufactured for:
Made in Hungary Rev. 3/16 100242
11PRINCIPAL DISPLAY PANEL - 40 mg BOTTLE

NDC 70515-629-30
Altoprev
(lovastatin)
extended-release tablets
(lovastatin)
extended-release tablets
40 mg
Rx only
COVIS
Each tablet contains lovastatin 40 mg.
Read package insert for prescribing
KEEP OUT OF THE REACH OF CHILDREN.
Dispense in tight, light-resistant container
Store at 20°-25°C (68°-77°F). Excursions
Avoid excessive heat and humidity.
Manufactured for:
Made in Hungary Rev. 3/16 100243
12PRINCIPAL DISPLAY PANEL - 60 mg BOTTLE

NDC 70515-630-30
Altoprev
(lovastatin)
extended-release tablets
(lovastatin)
extended-release tablets
60 mg
Rx only
COVIS
Each tablet contains lovastatin 60 mg.
Read package insert for prescribing
KEEP OUT OF THE REACH OF CHILDREN.
Dispense in tight, light-resistant container
Store at 20°-25°C (68°-77°F). Excursions
Avoid excessive heat and humidity.
Manufactured for:
Made in Hungary Rev. 3/16 100244