Generic Name
Everolimus
Brand Names
Torpenz, Zortress, Afinitor Disperz, Afinitor
FDA approval date: March 31, 2009
Classification: mTOR Inhibitor Immunosuppressant
Form: Tablet
What is Torpenz (Everolimus)?
TORPENZ tablets are a kinase inhibitor indicated for the treatment of: Postmenopausal women with advanced hormone receptor-positive, HER2-negative breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
TORPENZ (Everolimus)
1INDICATIONS AND USAGE
1.1 Hormone Receptor-Positive, HER2-Negative Breast Cancer
TORPENZ tablets are indicated for the treatment of postmenopausal women with advanced hormone receptor-positive, HER2-negative breast cancer in combination with exemestane, after failure of treatment with letrozole or anastrozole.
1.1Tuberous Sclerosis Complex (TSC)-Associated Renal Angiomyolipoma
TORPENZ tablets are indicated for the treatment of adult patients with renal angiomyolipoma and TSC, not requiring immediate surgery.
1.2Tuberous Sclerosis Complex (TSC)-Associated Subependymal Giant Cell Astrocytoma (SEGA)
TORPENZ tablets are indicated in adult and pediatric patients aged 1 year and older with TSC for the treatment of SEGA that requires therapeutic intervention but cannot be curatively resected.
2DOSAGE FORMS AND STRENGTHS
TORPENZ tablets are available containing 2.5 mg, 5 mg, 7.5 mg or 10 mg of everolimus.
- The
- The
- The
- The
3CONTRAINDICATIONS
TORPENZ tablets are contraindicated in patients with clinically significant hypersensitivity to everolimus or to other rapamycin derivatives
4ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Non-Infectious Pneumonitis
- Infections
- Severe Hypersensitivity Reactions
- Angioedema with Concomitant Use of ACE inhibitors
- Stomatitis
- Renal Failure
- Impaired Wound Healing
- Metabolic Disorders
- Myelosuppression
- Radiation Sensitization and Radiation Recall
4.1Post marketing Experience
The following adverse reactions have been identified during post approval use of everolimus. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate frequency or establish a causal relationship to drug exposure:
- Blood and Lymphatic Disorders:Thrombotic microangiopathy
- Cardiac:Cardiac failure with some cases reported with pulmonary hypertension (including pulmonary arterial hypertension) as a secondary event
- Gastrointestinal:Acute pancreatitis
- Hepatobiliary:Cholecystitis and cholelithiasis
- Infections:Sepsis and septic shock
- Nervous System:Reflex sympathetic dystrophy
- Vascular:Arterial thrombotic events, lymphedema
- Injury, Poisoning and Procedural Complications:Radiation sensitization and radiation recall
4.2Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates in other trials and may not reflect the rates observed in clinical practice.
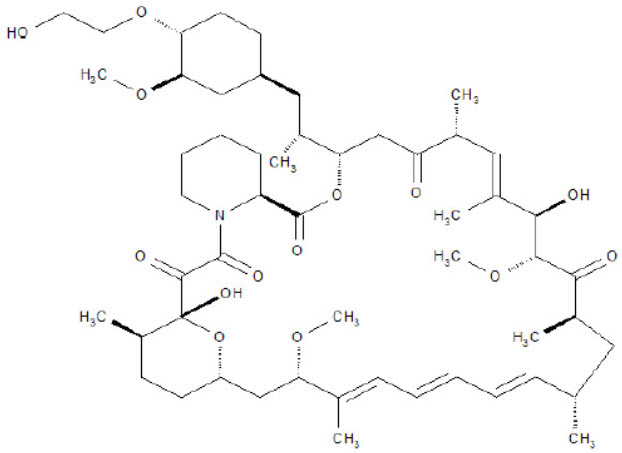
5DESCRIPTION
TORPENZ (everolimus) tablets are a kinase inhibitor.
The chemical name of everolimus is (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-1,18- dihydroxy-12-{(1R)-2-[(1S,3R,4R)-4-(2-hydroxyethoxy)-3-methoxycyclohexyl]-1-methylethyl}-19,30-dimethoxy- 15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0
TORPENZ tablets for oral administration contains 2.5 mg, 5 mg, 7.5 mg, or 10 mg of everolimus and the following inactive ingredients: anhydrous lactose, butylated hydroxytoluene, crospovidone, hypromellose, lactose monohydrate, and magnesium stearate.
6NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Administration of everolimus for up to 2 years did not indicate oncogenic potential in mice and rats up to the highest doses tested (0.9 mg/kg) corresponding, respectively to 3.9 and 0.2 times the estimated human exposure based on AUC at the recommended dose of everolimus tablets 10 mg orally once daily.
Everolimus was not genotoxic in a battery of in vitroassays (Ames mutation test in Salmonella, mutation test in L5178Y mouse lymphoma cells, and chromosome aberration assay in V79 Chinese hamster cells). Everolimus was not genotoxic in an in vivomouse bone marrow micronucleus test at doses up to 500 mg/kg/day (1,500 mg/m 2/day, approximately 255-fold the recommended dose of everolimus tablets 10 mg orally once daily, and approximately 200-fold the median dose administered to patients with TSC-associated SEGA, based on the BSA), administered as 2 doses, 24 hours apart.
Based on non-clinical findings, TORPENZ may impair male fertility. In a 13-week male fertility study in rats, testicular morphology was affected at doses of 0.5 mg/kg and above. Sperm motility, sperm count, and plasma testosterone levels were diminished in rats treated with 5 mg/kg. The exposures at these doses (52 ng∙hr/mL and 414 ng∙hr/mL, respectively) were within the range of human exposure at the recommended dose of everolimus tablets 10 mg orally once daily (560 ng∙hr/mL) and resulted in infertility in the rats at 5 mg/kg. Effects on male fertility occurred at AUC 0–24hvalues 10% to 81% lower than human exposure at the recommended dose of everolimus tablets 10 mg orally once daily. After a 10 to 13 week non-treatment period, the fertility index increased from zero (infertility) to 60%.
Oral doses of everolimus in female rats at doses ≥ 0.1 mg/kg (approximately 4% the human exposure based on AUC at the recommended dose of everolimus tablets 10 mg orally once daily) resulted in increased incidence of pre-implantation loss, suggesting that the drug may reduce female fertility.
6.1Animal Toxicology and/or Pharmacology
In juvenile rat toxicity studies, dose-related delayed attainment of developmental landmarks, including delayed eye-opening, delayed reproductive development in males and females and increased latency time during the learning and memory phases were observed at doses as low as 0.15 mg/kg/day.
7CLINICAL STUDIES
14.1 Hormone Receptor-Positive, HER2-Negative Breast Cancer
A randomized, double-blind, multicenter study (BOLERO-2, NCT00863655) of everolimus tablets in combination with exemestane vs. placebo in combination with exemestane was conducted in 724 postmenopausal women with estrogen receptor-positive, HER2-negative advanced breast cancer with recurrence or progression following prior therapy with letrozole or anastrozole. Randomization was stratified by documented sensitivity to prior hormonal therapy (yes vs. no) and by the presence of visceral metastasis (yes vs. no). Sensitivity to prior hormonal therapy was defined as either (1) documented clinical benefit (complete response [CR], partial response [PR], stable disease ≥ 24 weeks) to at least one prior hormonal therapy in the advanced setting or (2) at least 24 months of adjuvant hormonal therapy prior to recurrence. Patients were permitted to have received 0 to 1 prior lines of chemotherapy for advanced disease. The major efficacy outcome measure was progression-free survival (PFS) evaluated by RECIST (Response Evaluation Criteria in Solid Tumors), based on investigator (local radiology) assessment. Other outcome measures included overall survival (OS) and objective response rate (ORR).
Patients were randomized 2:1 to everolimus tablets 10 mg orally once daily in combination with exemestane 25 mg once daily (n = 485) or to placebo in combination with exemestane 25 mg orally once daily (n = 239). The two treatment groups were generally balanced with respect to baseline demographics and disease characteristics. Patients were not permitted to cross over to everolimus tablets at the time of disease progression.
The trial demonstrated a statistically significant improvement in PFS by investigator assessment (Table 20 and Figure 1). The results of the PFS analysis based on independent central radiological assessment were consistent with the investigator assessment. PFS results were also consistent across the subgroups of age, race, presence and extent of visceral metastases, and sensitivity to prior hormonal therapy.
ORR was higher in the everolimus tablets in combination with exemestane arm vs. the placebo in combination with exemestane arm (Table 20). There were 3 complete responses (0.6%) and 58 partial responses (12%) in the everolimus tablets arm. There were no complete responses and 4 partial responses (1.7%) in the placebo in combination with exemestane arm.
After a median follow-up of 39.3 months, there was no statistically significant difference in OS between the everolimus tablets in combination with exemestane arm and the placebo in combination with exemestane arm [HR 0.89 (95% CI: 0.73, 1.10)].
7.1Tuberous Sclerosis Complex (TSC)-Associated Renal Angiomyolipoma
A randomized (2:1), double-blind, placebo-controlled trial (EXIST-2, NCT00790400) of everolimus tablets was conducted in 118 patients with renal angiomyolipoma as a feature of TSC (n = 113) or sporadic lymphangioleiomyomatosis (n = 5). The key eligibility requirements for this trial were at least one angiomyolipoma of ≥ 3 cm in longest diameter on CT/MRI based on local radiology assessment, no immediate indication for surgery, and age ≥ 18 years. Patients received everolimus tablets 10 mg or matching placebo orally once daily until disease progression or unacceptable toxicity. CT or MRI scans for disease assessment were obtained at baseline, 12, 24, and 48 weeks and annually thereafter. Clinical and photographic assessment of skin lesions were conducted at baseline and every 12 weeks thereafter until treatment discontinuation. The major efficacy outcome measure was angiomyolipoma response rate based on independent central radiology review, which was defined as a ≥ 50% reduction in angiomyolipoma volume, absence of new angiomyolipoma lesion ≥ 1 cm, absence of kidney volume increase ≥ 20%, and no angiomyolipoma related bleeding of ≥ Grade 2. Key supportive efficacy outcome measures were time to angiomyolipoma progression and skin lesion response rate. The primary analyses of efficacy outcome measures were limited to the blinded treatment period and conducted 6 months after the last patient was randomized. The comparative angiomyolipoma response rate analysis was stratified by use of enzyme-inducing antiepileptic drugs (EIAEDs) at randomization (yes vs. no).
Of the 118 patients enrolled, 79 were randomized to everolimus tablets and 39 to placebo. The median age was 31 years (18 to 61 years), 34% were male, and 89% were white. At baseline, 17% of patients were receiving EIAEDs. On central radiology review at baseline, 92% of patients had at least 1 angiomyolipoma of ≥ 3 cm in longest diameter, 29% had angiomyolipomas ≥ 8 cm, 78% had bilateral angiomyolipomas, and 97% had skin lesions. The median values for the sum of all target renal angiomyolipoma lesions at baseline were 85 cm
There were 3 patients in the everolimus tablets arm and 8 patients in the placebo arm with documented angiomyolipoma progression by central radiologic review (defined as a ≥ 25% increase from nadir in the sum of angiomyolipoma target lesion volumes to a value greater than baseline, appearance of a new angiomyolipoma ≥ 1 cm in longest diameter, an increase in renal volume ≥ 20% from nadir for either kidney and to a value greater than baseline, or Grade ≥ 2 angiomyolipoma-related bleeding). The time to angiomyolipoma progression was statistically significantly longer in the everolimus tablets arm (HR 0.08 [95% CI: 0.02, 0.37]; p < 0.0001).
Skin lesion response rates were assessed by local investigators for 77 patients in the everolimus tablets arm and 37 patients in the placebo arm who presented with skin lesions at study entry. The skin lesion response rate was statistically significantly higher in the everolimus tablets arm (26% vs. 0, p = 0.0011); all skin lesion responses were partial responses, defined as visual improvement in 50% to 99% of all skin lesions durable for at least 8 weeks (Physician's Global Assessment of Clinical Condition).
Patients randomized to placebo were permitted to receive everolimus tablets at the time of angiomyolipoma progression or after the time of the primary analysis. After the primary analysis, patients treated with everolimus tablets underwent additional follow-up CT or MRI scans to assess tumor status until discontinuation of treatment or completion of 4 years of follow-up after the last patient was randomized. A total of 112 patients (79 randomized to everolimus tablets and 33 randomized to placebo) received at least one dose of everolimus tablets. The median duration of everolimus tablets treatment was 3.9 years (0.5 months to 5.3 years) and the median duration of follow-up was 3.9 years (0.9 months to 5.4 years). During the follow-up period after the primary analysis, 32 patients (in addition to the 33 patients identified at the time of the primary analysis) had an angiomyolipoma response based upon independent central radiology review. Among the 65 responders out of 112 patients, the median time to angiomyolipoma response was 2.9 months (2.6 to 33.8 months). Fourteen percent of the 112 patients treated with everolimus tablets had angiomyolipoma progression by the end of the follow-up period. No patient underwent a nephrectomy for angiomyolipoma progression, and one patient underwent renal embolization while treated with everolimus tablets.
8REFERENCES
- OSHA Hazardous Drugs.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
10HOW SUPPLIED/STORAGE AND HANDLING
TORPENZ tablets for oral use are available containing 2.5 mg, 5 mg, 7.5 mg, or 10 mg of everolimus.
The 2.5 mg tablets are white to off white, capsule shaped, flat faced, beveled edged tablets debossed with "B 2.5" on one side and plain on the other side. They are supplied as follows:
Bottles of 30 with a child-resistant closure, NDC 0245-0822-30
Unit-Dose cartons of 28, NDC 0245-0822-04
(4 blister cards with embedded desiccants containing 7 tablets each)
The 5 mg tablets are white to off white, capsule shaped, flat faced, beveled edged tablets debossed with "B 5" on one side and plain on the other side. They are supplied as follows:
Bottles of 30 with a child-resistant closure, NDC 0245-0823-30
Unit-Dose cartons of 28, NDC 0245-0823-04
(4 blister cards with embedded desiccants containing 7 tablets each)
The 7.5 mg tablets are white to off white, capsule shaped, flat faced, beveled edged tablets debossed with "B 7.5" on one side and plain on the other side. They are supplied as follows:
Bottles of 30 with a child-resistant closure, NDC 0245-0824-30
Unit-Dose cartons of 28, NDC 0245-0824-04
(4 blister cards with embedded desiccants containing 7 tablets each)
The 10 mg tablets are white to off white, capsule shaped, flat faced, beveled edged tablets debossed with "B 10" on one side and plain on the other side. They are supplied as follows:
Bottles of 30 with a child-resistant closure, NDC 0245-0825-30
Unit-Dose cartons of 28, NDC 0245-0825-04
(4 blister cards with embedded desiccants containing 7 tablets each)
11Patient Information
This Patient Information has been approved by the U.S. Food and Drug Administration.
Made in India
Manufactured for
Revised: 5/2025
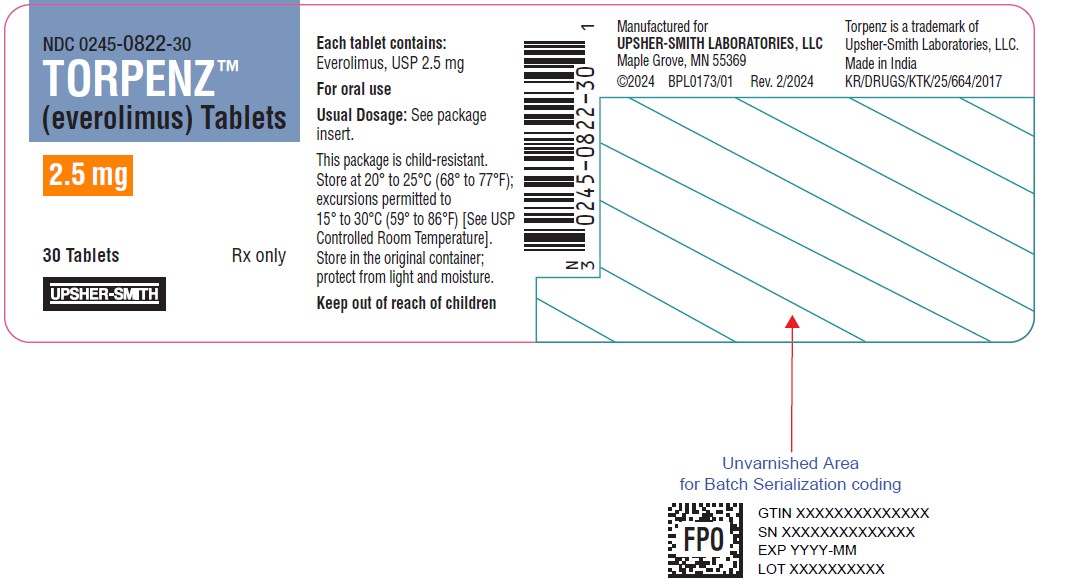
12PRINCIPAL DISPLAY PANEL - 2.5 mg Tablet Bottle Label
NDC 0245-0822-30
TORPENZ™
2.5 mg
30 Tablets
UPSHER-SMITH
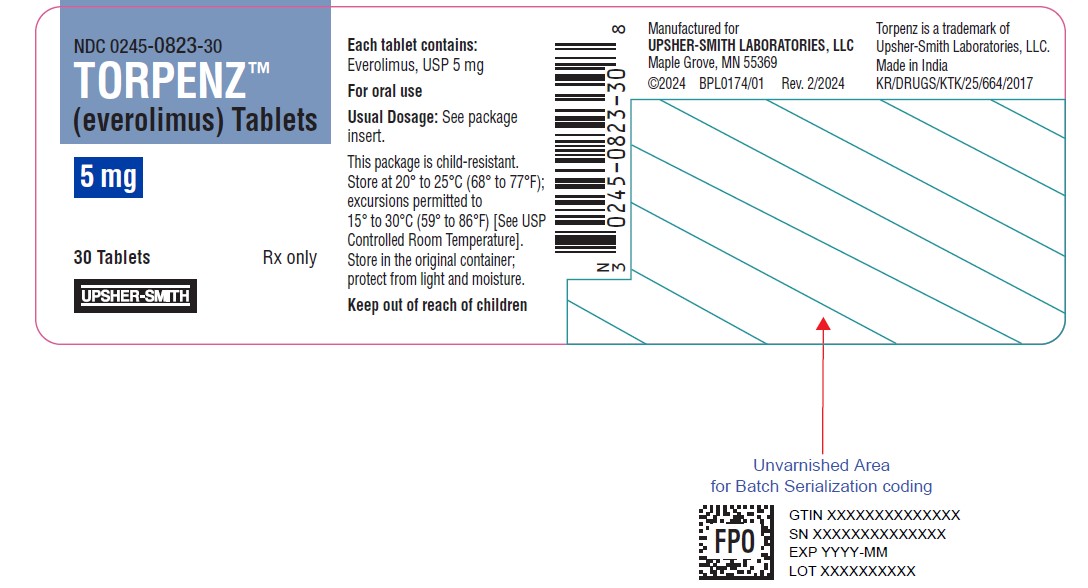
13PRINCIPAL DISPLAY PANEL - 5 mg Tablet Bottle Label
NDC 0245-0823-30
TORPENZ™
5 mg
30 Tablets
UPSHER-SMITH
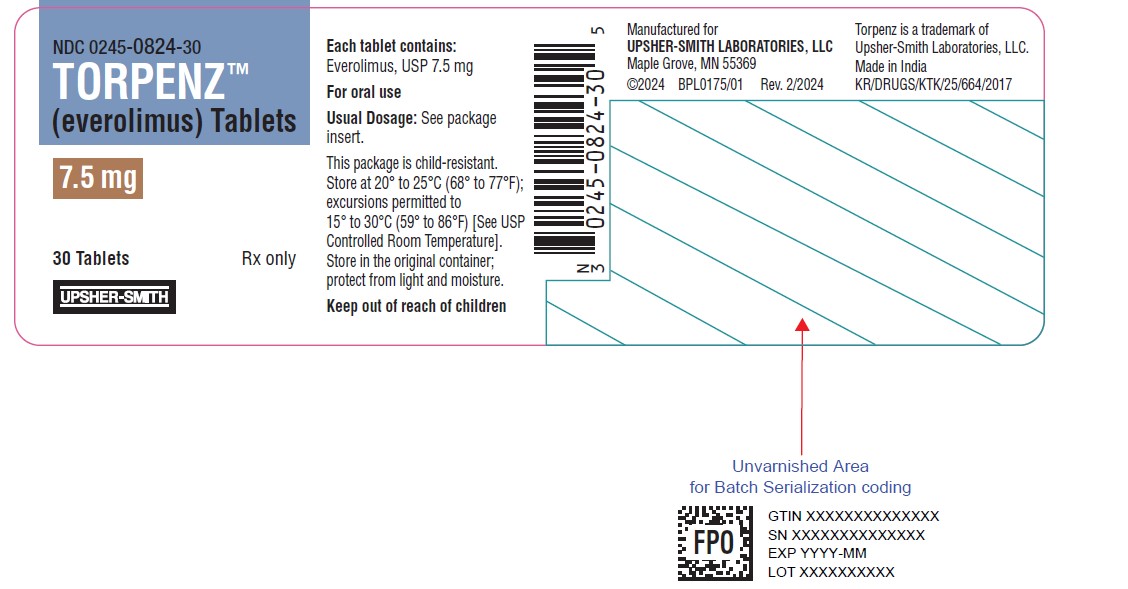
14PRINCIPAL DISPLAY PANEL - 7.5 mg Tablet Bottle Label
NDC 0245-0824-30
TORPENZ™
7.5 mg
30 Tablets
UPSHER-SMITH
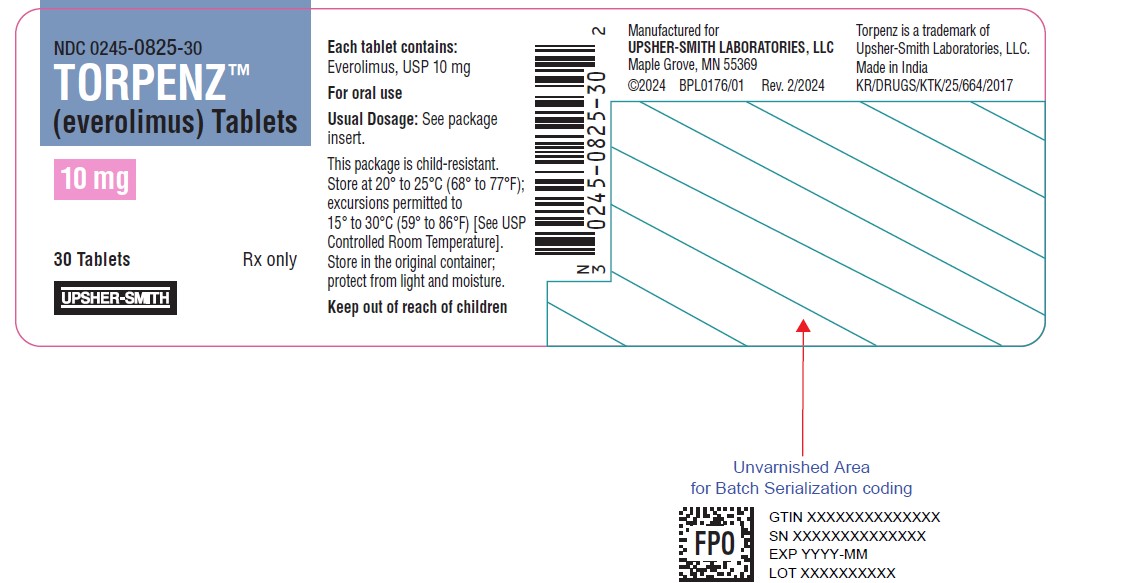
15PRINCIPAL DISPLAY PANEL - 10 mg Tablet Bottle Label
NDC 0245-0825-30
TORPENZ™
10 mg
30 Tablets
UPSHER-SMITH