Brand Name
Effexor
Generic Name
Venlafaxine
View Brand Information FDA approval date: November 01, 1997
Classification: Serotonin and Norepinephrine Reuptake Inhibitor
Form: Tablet, Capsule
What is Effexor (Venlafaxine)?
Venlafaxine Hydrochloride Extended-Release Capsules, USP are indicated in adults for the treatment of: Major Depressive Disorder [see Clinical Studies (1.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Effexor (VENLAFAXINE HYDROCHLORIDE)
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behavior in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and emergence of suicidal thoughts and behaviors Warnings and Precautions (5.1)]. Effexor XR is not approved for use in pediatric patients Use in Specific Populations (8.4)].
1INDICATIONS AND USAGE
Effexor XR is indicated in adults for the treatment of:
- Major Depressive Disorder (MDD)
- Generalized Anxiety Disorder (GAD)
- Social Anxiety Disorder (SAD)
- Panic Disorder (PD)
2DOSAGE FORMS AND STRENGTHS
Effexor XR
- 37.5 mg extended-release capsule: grey cap and peach body with "W" and "Effexor XR" on the cap and "37.5" on the body
- 75 mg extended-release capsule: peach cap and body with "W" and "Effexor XR" on the cap and "75" on the body
- 150 mg extended-release capsule: dark orange cap and body with "W" and "Effexor XR" on the cap and "150" on the body
3CONTRAINDICATIONS
Effexor XR is contraindicated in patients:
- with known hypersensitivity to venlafaxine hydrochloride, desvenlafaxine succinate or to any excipients in the formulation
- taking, or within 14 days of stopping, MAOIs (including the MAOIs linezolid and intravenous methylene blue) because of the risk of serotonin syndrome
4ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Hypersensitivity
- Suicidal Thoughts and Behaviors in Adolescents and Young Adults
- Serotonin Syndrome
- Elevated Blood Pressure
- Increased Risk of Bleeding
- Angle-Closure Glaucoma
- Activation of Mania/Hypomania
- Discontinuation Syndrome
- Seizure
- Hyponatremia
- Weight and Height changes in Pediatric Patients
- Appetite Changes in Pediatric Patients
- Interstitial Lung Disease and Eosinophilic Pneumonia
- Sexual Dysfunction
4.1Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Effexor XR. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a whole – Anaphylaxis, angioedema
Cardiovascular system – QT prolongation, ventricular fibrillation, ventricular tachycardia (including torsade de pointes), takotsubo cardiomyopathy
Digestive system – Pancreatitis
Hemic/Lymphatic system – Mucous membrane bleeding [see , blood dyscrasias (including agranulocytosis, aplastic anemia, neutropenia and pancytopenia), prolonged bleeding time, thrombocytopenia
Metabolic/Nutritional – Hyponatremia [see , Syndrome of Inappropriate Antidiuretic Hormone (SIADH) secretion [see , abnormal liver function tests, hepatitis, prolactin increased
Musculoskeletal – Rhabdomyolysis
Nervous system – Neuroleptic Malignant Syndrome (NMS) [see , serotonergic syndrome [see , delirium, extrapyramidal reactions (including dystonia and dyskinesia), impaired coordination and balance, tardive dyskinesia
Respiratory system – Dyspnea, interstitial lung disease, pulmonary eosinophilia [see
Skin and appendages – Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme
Special senses – Angle-closure glaucoma [see
5DESCRIPTION
Effexor XR is an extended-release capsule for once-a-day oral administration that contains venlafaxine hydrochloride, a serotonin and norepinephrine reuptake inhibitor (SNRI).
Venlafaxine is designated (R/S)-1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl] cyclohexanol hydrochloride or (±)-1-[α- [(dimethylamino)methyl]-p-methoxybenzyl] cyclohexanol hydrochloride and has the empirical formula of C
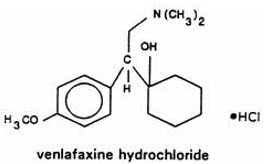
Venlafaxine hydrochloride is a white to off-white crystalline solid, with a solubility of 572 mg/mL in water (adjusted to ionic strength of 0.2 M with sodium chloride). Its octanol:water (0.2 M sodium chloride) partition coefficient is 0.43.
Drug release is controlled by diffusion through the coating membrane on the spheroids and is not pH-dependent. Capsules contain venlafaxine hydrochloride equivalent to 37.5 mg, 75 mg, or 150 mg venlafaxine. Inactive ingredients consist of cellulose, ethylcellulose, gelatin, hypromellose, iron oxide, and titanium dioxide.
6HOW SUPPLIED/STORAGE AND HANDLING
Effexor XR
- 37.5 mg, grey cap/peach body with "W" and "Effexor XR" on the cap and "37.5" on the body.
- 75 mg, peach cap and body with "W" and "Effexor XR" on the cap and "75" on the body.
- 150 mg, dark orange cap and body with "W" and "Effexor XR" on the cap and "150" on the body.
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
8PRINCIPAL DISPLAY PANEL - 37.5 mg Capsule Bottle Label
ALWAYS DISPENSE WITH MEDICATION GUIDE
Pfizer
Effexor XR
37.5 mg*
30 Capsules

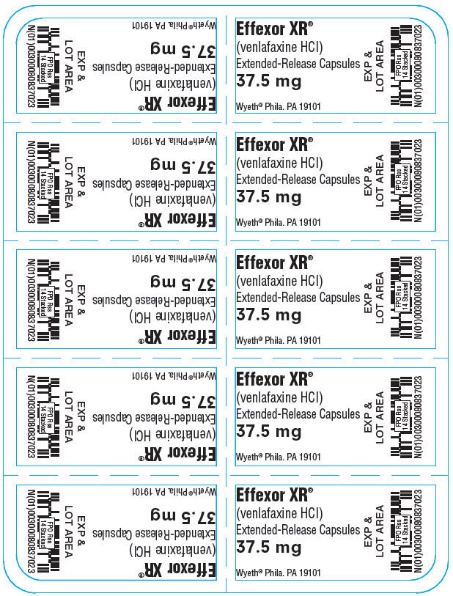
9PRINCIPAL DISPLAY PANEL - 37.5 mg Capsule Blister Pack
Effexor XR
(venlafaxine HCl)
Extended-Release Capsules
37.5 mg
(venlafaxine HCl)
Extended-Release Capsules
37.5 mg
Wyeth
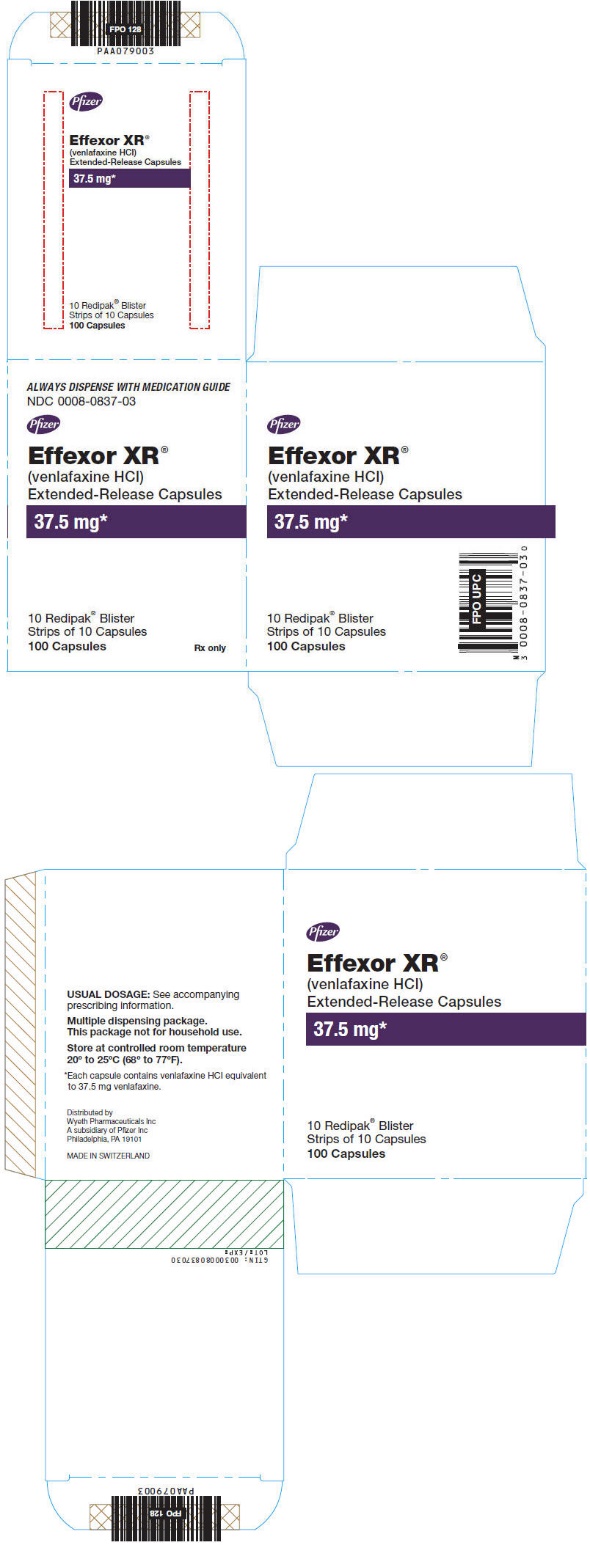
10PRINCIPAL DISPLAY PANEL - 37.5 mg Capsule Blister Pack Carton
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0008-0837-03
NDC 0008-0837-03
Pfizer
Effexor XR®
(venlafaxine HCl)
Extended-Release Capsules
(venlafaxine HCl)
Extended-Release Capsules
37.5 mg*
10 Redipak
Rx only
11PRINCIPAL DISPLAY PANEL - 75 mg Capsule Bottle Label
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0008-0833-21
Pfizer
Effexor XR
75 mg*
30 Capsules

12PRINCIPAL DISPLAY PANEL - 75 mg Capsule Blister Pack
Effexor XR
(venlafaxine HCl)
Extended-Release Capsules
75 mg
(venlafaxine HCl)
Extended-Release Capsules
75 mg
Wyeth
13PRINCIPAL DISPLAY PANEL - 75 mg Capsule Blister Pack Carton
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0008-0833-03
NDC 0008-0833-03
Pfizer
Effexor XR®
(venlafaxine HCl)
Extended-Release Capsules
(venlafaxine HCl)
Extended-Release Capsules
75 mg*
10 Redipak
Rx only

14PRINCIPAL DISPLAY PANEL - 150 mg Capsule Bottle Label
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0008-0836-21
Pfizer
Effexor XR
150 mg*
30 Capsules

15PRINCIPAL DISPLAY PANEL - 150 mg Capsule Blister Pack
Effexor XR
(venlafaxine HCl)
Extended-Release Capsules
150 mg
(venlafaxine HCl)
Extended-Release Capsules
150 mg
Wyeth
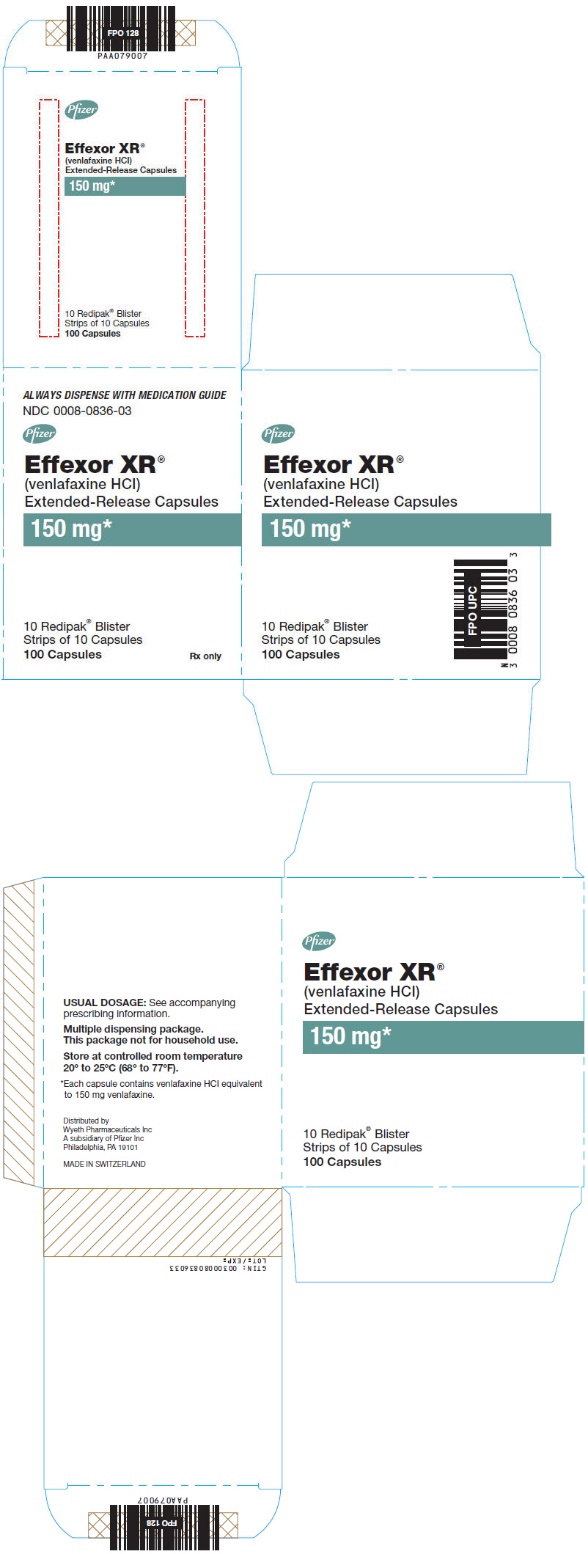
16PRINCIPAL DISPLAY PANEL - 150 mg Capsule Blister Pack Carton
ALWAYS DISPENSE WITH MEDICATION GUIDE
NDC 0008-0836-03
NDC 0008-0836-03
Pfizer
Effexor XR
(venlafaxine HCl)
Extended-Release Capsules
(venlafaxine HCl)
Extended-Release Capsules
150 mg*
10 Redipak
Rx only