Brand Name
Dexilant
Generic Name
Dexlansoprazole
View Brand Information FDA approval date: April 12, 2010
Classification: Proton Pump Inhibitor
Form: Capsule
What is Dexilant (Dexlansoprazole)?
Dexlansoprazole delayed-release capsules are a proton pump inhibitor indicated in patients 12 years of age and older for: Healing of all grades of erosive esophagitis .
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Dexilant (dexlansoprazole)
1DOSAGE FORMS AND STRENGTHS
DEXILANT delayed-release capsules
- 30 mg: strength is an opaque, blue and gray capsule imprinted with "TAP" and "30".
- 60 mg: strength is an opaque, blue capsule imprinted with "TAP" and "60".
2CONTRAINDICATIONS
- DEXILANT is contraindicated in patients with known hypersensitivity to any component of the formulation
- PPIs, including DEXILANT, are contraindicated with rilpivirine-containing products
3ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in labeling:
- Acute Tubulointerstitial Nephritis
- Clostridium difficile-Associated Diarrhea [see
- Bone Fracture
- Severe Cutaneous Adverse Reactions
- Cutaneous and Systemic Lupus Erythematosus
- Cyanocobalamin (Vitamin B12) Deficiency
- Hypomagnesemia and Mineral Metabolism
- Fundic Gland Polyps
- Risk of Heart Valve Thickening in Pediatric Patients Less than Two Years of Age
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
3.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of DEXILANT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: autoimmune hemolytic anemia, idiopathic thrombocytopenic purpura
Ear and Labyrinth Disorders: deafness
Eye Disorders: blurred vision
Gastrointestinal Disorders: oral edema, pancreatitis, fundic gland polyps
General Disorders and Administration Site Conditions: facial edema
Hepatobiliary Disorders: drug-induced hepatitis
Immune System Disorders: anaphylactic shock (requiring emergency intervention), exfoliative dermatitis, SJS/TEN (some fatal), DRESS, AGEP, erythema multiforme
Infections and Infestations: Clostridium difficile-associated diarrhea
Metabolism and Nutrition Disorders: hypomagnesemia, hypocalcemia, hypokalemia, hyponatremia
Musculoskeletal System Disorders: bone fracture
Nervous System Disorders: cerebrovascular accident, transient ischemic attack
Renal and Genitourinary Disorders: acute renal failure, erectile dysfunction
Respiratory, Thoracic and Mediastinal Disorders: pharyngeal edema, throat tightness
Skin and Subcutaneous Tissue Disorders: generalized rash, leukocytoclastic vasculitis
4DRUG INTERACTIONS
Tables 3 and 4 include drugs with clinically important drug interactions and interaction with diagnostics when administered concomitantly with DEXILANT and instructions for preventing or managing them.
Consult the labeling of concomitantly used drugs to obtain further information about interactions with PPIs.
5OVERDOSAGE
There have been no reports of significant overdose with DEXILANT. Multiple doses of DEXILANT 120 mg and a single dose of DEXILANT 300 mg did not result in death or other severe adverse events. However, serious adverse events of hypertension have been reported in association with twice daily doses of DEXILANT 60 mg. Nonserious adverse reactions observed with twice daily doses of DEXILANT 60 mg include hot flashes, contusion, oropharyngeal pain, and weight loss. Dexlansoprazole is not expected to be removed from the circulation by hemodialysis.
In the event of over-exposure, treatment should be symptomatic and supportive.
If over-exposure occurs, call your poison control center at 1-800-222-1222 for current information on the management of poisoning or over-exposure.
6DESCRIPTION
The active ingredient in DEXILANT (dexlansoprazole) delayed-release capsules, a proton pump inhibitor, is (+)-2-[(
Dexlansoprazole has the following chemical structure:
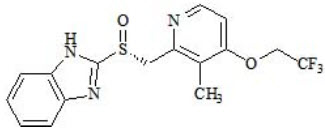
Dexlansoprazole is a white to nearly white crystalline powder which melts with decomposition at 140°C. Dexlansoprazole is freely soluble in dimethylformamide, methanol, dichloromethane, ethanol, and ethyl acetate; and soluble in acetonitrile; slightly soluble in ether; and very slightly soluble in water; and practically insoluble in hexane.
Dexlansoprazole is stable when exposed to light. Dexlansoprazole is more stable in neutral and alkaline conditions than acidic conditions.
Dexlansoprazole is supplied for oral administration as a dual delayed-release formulation in capsules. The capsules contain dexlansoprazole in a mixture of two types of enteric-coated granules with different pH-dependent dissolution profiles
DEXILANT delayed-release capsules are available in two dosage strengths: 30 and 60 mg, per capsule. Each capsule contains enteric-coated granules consisting of dexlansoprazole (active ingredient) and the following inactive ingredients: sugar spheres, magnesium carbonate, sucrose, low-substituted hydroxypropyl cellulose, titanium dioxide, hydroxypropyl cellulose, hypromellose 2910, talc, methacrylic acid copolymers, polyethylene glycol 8000, triethyl citrate, polysorbate 80, and colloidal silicon dioxide. The components of the capsule shell include the following inactive ingredients: hypromellose, carrageenan and potassium chloride. Based on the capsule shell color, blue contains FD&C Blue No. 2 (or FD&C Blue No. 2 aluminum lake); gray contains black ferric oxide; and both contain titanium dioxide.
7HOW SUPPLIED/STORAGE AND HANDLING
DEXILANT delayed-release capsules, 30 mg, are opaque, blue and gray with "TAP" and "30" imprinted on the capsule and supplied as:
DEXILANT delayed-release capsules, 60 mg, are opaque, blue with "TAP" and "60" imprinted on the capsule and supplied as:
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
9INSTRUCTIONS FOR USE
DEXILANT
Taking DEXILANT with applesauce:
- Place 1 tablespoon of applesauce into a clean container.
- Carefully open the capsule and sprinkle the granules onto the applesauce.
- Swallow the applesauce and granules right away. Do not chew the granules. Do not save the applesauce and granules for later use.
Giving DEXILANT with water using an oral syringe:
- Place 20 mL of water into a clean container.
- Carefully open the capsule and empty the granules into the container of water.
- Use an oral syringe to draw up the water and granule mixture.
- Gently swirl the oral syringe to keep the granules from settling.
- Place the tip of the oral syringe in your mouth. Give the medicine right away. Do not save the water and granule mixture for later use.
- Refill the syringe with 10 mL of water and swirl gently. Place the tip of the oral syringe in your mouth and give the medicine that is left in the syringe.
- Repeat step 6.
Giving DEXILANT with water through a nasogastric tube (NG tube):
For people who have an NG tube that is
- Place 20 mL of water into a clean container.
- Carefully open the capsule and empty the granules into the container of water.
- Use a 60 mL catheter-tip syringe to draw up the water and granule mixture.
- Gently swirl the catheter-tip syringe to keep the granules from settling.
- Connect the catheter-tip syringe to the NG tube.
- Give the mixture right away through the NG tube that goes into the stomach. Do not save the water and granule mixture for later use.
- Refill the catheter-tip syringe with 10 mL of water and swirl gently. Flush the NG tube with the water.
- Repeat step 7.
How should I store DEXILANT?
- Store DEXILANT at room temperature between 68°F to 77°F (20°C to 25°C).
Keep DEXILANT and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by:
DEXILANT and
© 2025 Takeda Pharmaceuticals America, Inc. All rights reserved.
Revised: February 2025
DEX006 R37
10PRINCIPAL DISPLAY PANEL - 30 mg Capsule Bottle Label
NDC 64764-171-30
Rx only
30 Capsules
30 Capsules
DEXILANT
Dispense the
30 mg

11PRINCIPAL DISPLAY PANEL - 60 mg Capsule Bottle Label
NDC 64764-175-30
Rx only
30 Capsules
30 Capsules
DEXILANT
Dispense the
60 mg
