Brand Name
Avodart
Generic Name
Dutasteride
View Brand Information FDA approval date: November 02, 2015
Classification: 5-alpha Reductase Inhibitor
Form: Capsule
What is Avodart (Dutasteride)?
Dutasteride is a 5 alpha-reductase inhibitor indicated for the treatment of symptomatic benign prostatic hyperplasia in men with an enlarged prostate to.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
AVODART (dutasteride)
1DOSAGE AND ADMINISTRATION
The capsules should be swallowed whole and not chewed or opened, as contact with the capsule contents may result in irritation of the oropharyngeal mucosa. AVODART may be administered with or without food.
1.1Monotherapy
The recommended dose of AVODART is 1 capsule (0.5 mg) taken once daily.
1.2Combination with Alpha-adrenergic Antagonist
The recommended dose of AVODART is 1 capsule (0.5 mg) taken once daily and tamsulosin 0.4 mg taken once daily.
2DOSAGE FORMS AND STRENGTHS
0.5-mg, opaque, dull yellow, gelatin capsules imprinted with “GX CE2” in red ink on one side.
3CONTRAINDICATIONS
AVODART is contraindicated for use in:
- Pregnancy. Dutasteride use is contraindicated in women who are pregnant. In animal reproduction and developmental toxicity studies, dutasteride inhibited development of male fetus external genitalia. Therefore, AVODART may cause fetal harm when administered to a pregnant woman
- Patients with previously demonstrated clinically significant hypersensitivity (e.g., serious skin reactions, angioedema) to AVODART or other 5 alpha-reductase inhibitors
4OVERDOSAGE
In volunteer trials, single doses of dutasteride up to 40 mg (80 times the therapeutic dose) for 7 days have been administered without significant safety concerns. In a clinical trial, daily doses of 5 mg (10 times the therapeutic dose) were administered to 60 subjects for 6 months with no additional adverse effects to those seen at therapeutic doses of 0.5 mg.
There is no specific antidote for dutasteride. Therefore, in cases of suspected overdosage, symptomatic and supportive treatment should be given as appropriate, taking the long half-life of dutasteride into consideration.
5DESCRIPTION
AVODART is a synthetic 4-azasteroid compound that is a selective inhibitor of both the type 1 and type 2 isoforms of steroid 5 alpha-reductase, an intracellular enzyme that converts testosterone to DHT.
Dutasteride is chemically designated as (5α,17β)-N-{2,5 bis(trifluoromethyl)phenyl}-3-oxo-4-azaandrost-1-ene-17-carboxamide. The empirical formula of dutasteride is C
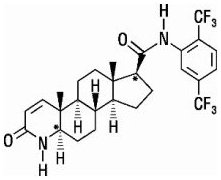
Dutasteride is a white to pale yellow powder with a melting point of 242° to 250°C. It is soluble in ethanol (44 mg/mL), methanol (64 mg/mL), and polyethylene glycol 400 (3 mg/mL), but it is insoluble in water.
Each AVODART soft gelatin capsule, administered orally, contains 0.5 mg of dutasteride dissolved in a mixture of mono-di-glycerides of caprylic/capric acid and butylated hydroxytoluene. The inactive excipients in the capsule shell are ferric oxide (yellow), gelatin (from certified BSE-free bovine sources), glycerin, and titanium dioxide. The soft gelatin capsules are printed with edible red ink.
6HOW SUPPLIED/STORAGE AND HANDLING
AVODART soft gelatin capsules 0.5 mg are oblong, opaque, dull yellow, gelatin capsules imprinted with “GX CE2” with red edible ink on one side, packaged in bottles of 30 (NDC 80725-712-15) and 90 (NDC 80725-712-04) with child-resistant closures.
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Dutasteride is absorbed through the skin. AVODART capsules should not be handled by women who are pregnant or who could become pregnant because of the potential for absorption of dutasteride and the subsequent potential risk to a developing male fetus
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
PSA Monitoring
Inform patients that AVODART reduces serum PSA levels by approximately 50% within 3 to 6 months of therapy, although it may vary for each individual. For patients undergoing PSA screening, increases in PSA levels while on treatment with AVODART may signal the presence of prostate cancer and should be evaluated by a healthcare provider
Increased Risk of High-grade Prostate Cancer
Inform patients that there was an increase in high-grade prostate cancer in men treated with 5 alpha-reductase inhibitors (which are indicated for BPH treatment), including AVODART, compared with those treated with placebo in trials looking at the use of these drugs to reduce the risk of prostate cancer
Transdermal Exposure of AVODART in Pregnant or Potentially Pregnant Women—Risk to Male Fetus
Inform patients that AVODART capsules should not be handled by women who are pregnant or may potentially be pregnant because of the potential for absorption of dutasteride and the subsequent potential risk to a developing male fetus. Dutasteride can be absorbed through the skin and could result in unintended fetal exposure. If a pregnant or potentially pregnant woman comes in contact with leaking AVODART capsules, the contact area should be washed immediately with soap and water
Effects on Semen Parameters
Advise men that AVODART may affect sperm characteristics but the effect on fertility is unknown
Blood Donation
Inform men treated with AVODART that they should not donate blood until at least 6 months following their last dose to prevent pregnant women from receiving dutasteride through blood transfusion
AVODART is a trademark used under license by Waylis Therapeutics LLC.
The other brands listed are trademarks owned by or licensed to their respective owners and are not owned by or licensed to Waylis Therapeutics LLC. The makers of these brands are not affiliated with and do not endorse Waylis Therapeutics LLC or its products.
Manufactured for:
Waylis Therapeutics LLC
PHARMACIST-DETACH HERE AND GIVE INSTRUCTIONS TO PATIENT
----------------------------------------------------------------------------------------------------------