Elfabrio
What is Elfabrio (Pegunigalsidase)?
Living with a rare genetic condition like Fabry disease can be challenging physically, emotionally and practically. Many patients experience pain, fatigue and organ complications that affect their daily lives. Elfabrio is a newer treatment option designed to help patients with Fabry disease manage these symptoms and protect long-term health.
Elfabrio belongs to a class of medications known as enzyme replacement therapies (ERTs). It replaces a missing or deficient enzyme in the body, helping to reduce the harmful buildup of certain substances that cause Fabry-related complications. Approved by the U.S. Food and Drug Administration (FDA) in 2023, Elfabrio offers a modern, long-acting option for individuals seeking effective and convenient management of this lifelong condition.
What does Elfabrio do?
Elfabrio is prescribed to treat Fabry disease, a rare inherited disorder caused by a deficiency of the enzyme alpha-galactosidase A. This enzyme is essential for breaking down a fatty substance called globotriaosylceramide (Gb3). When Gb3 accumulates in cells, it can damage the kidneys, heart, and nervous system over time.
By providing a long-lasting form of the missing enzyme, Elfabrio helps clear Gb3 buildup from the body’s cells. This action can:
- Reduce pain, fatigue and heat intolerance
- Help protect kidney and heart function
- Improve overall quality of life
Clinical studies have shown that Elfabrio is effective and well-tolerated, with sustained enzyme activity and reduced antibody formation compared to older Fabry treatments (FDA, 2023; Chiesi Global Rare Diseases, 2024).
Elfabrio is considered a specialized therapy, typically prescribed by genetic or metabolic specialists as a long-term treatment for Fabry disease in both adults and adolescents.
How does Elfabrio work?
Fabry disease occurs when the body lacks enough of the enzyme alpha-galactosidase A, leading to the buildup of fatty molecules (Gb3 and lyso-Gb3) inside cells. Over time, this buildup interferes with normal organ function.
Elfabrio (pegunigalsidase alfa) works by providing a synthetic form of the missing enzyme, engineered to closely mimic the body’s natural version. Once infused into the bloodstream, it is taken up by cells, where it breaks down Gb3 and other accumulated substances.
What makes Elfabrio unique is its pegylated formulation, meaning it’s chemically modified with polyethylene glycol (PEG) to help the enzyme last longer in the bloodstream. This allows for infusions every two weeks, improving convenience and reducing treatment fatigue for patients.
Clinically, this mechanism helps reduce pain, prevent organ damage, and stabilize disease progression allowing patients to maintain a more normal daily routine and improve physical endurance.
Elfabrio side effects
Like all medications, Elfabrio may cause side effects, though most are mild to moderate and manageable with medical supervision.
Common side effects include:
- Headache
- Fatigue
- Nausea or vomiting
- Chills or fever during or after infusion
- Muscle or joint pain
Less common side effects:
- Dizziness
- Abdominal discomfort
- Cough or nasal congestion
- Skin reactions (itching or redness at infusion site)
Serious side effects (rare):
- Severe allergic reactions (anaphylaxis)
- Infusion-related reactions such as swelling, difficulty breathing, or low blood pressure
- Formation of antibodies against the enzyme, which may reduce treatment effectiveness
Patients should notify their healthcare provider immediately if they experience shortness of breath, chest tightness, rash, or swelling of the face or throat.
Doctors may give premedications (like antihistamines or steroids) before infusions to prevent or minimize infusion reactions.
Who should avoid Elfabrio: Individuals with a known allergy to pegunigalsidase alfa or any ingredient in the formulation should not receive Elfabrio (FDA, 2023).
Overall, most patients tolerate Elfabrio well, especially when monitored regularly by their healthcare team.
Elfabrio dosage
Elfabrio is given as an intravenous (IV) infusion administered in a healthcare setting or, in some cases, through a home infusion program supervised by trained professionals.
Treatment is typically given once every two weeks, with each infusion lasting several hours. The exact schedule and duration depend on the patient’s weight, response and clinical condition.
Monitoring and follow-up are essential for safe and effective therapy. Doctors may:
- Check kidney and heart function regularly to assess disease progression
- Monitor antibody levels to ensure continued enzyme effectiveness
- Observe for any infusion-related reactions and adjust premedication as needed
- Evaluate pain and quality-of-life improvements over time
For patients with existing renal or cardiac involvement, or those transitioning from another enzyme replacement therapy, dose adjustments and closer monitoring may be recommended.
Does Elfabrio have a generic version?
Currently, Elfabrio (pegunigalsidase alfa-iwxj) does not have a generic version available. It is a biologic medication, meaning it is made from living cells rather than chemical compounds.
Because biologics are complex to manufacture, direct generics are not possible. However, in the future, a biosimilar version (a highly similar but not identical biologic) could be developed once the original patent exclusivity expires.
At present, Elfabrio is available only under its brand name, produced by Chiesi Global Rare Diseases.
Patients switching from other enzyme replacement therapies such as Fabrazyme (agalsidase beta) or Replagal (agalsidase alfa) should discuss transition plans carefully with their doctor to ensure continuity and safety.
Conclusion
Elfabrio (pegunigalsidase alfa-iwxj) represents an important advancement in the treatment of Fabry disease, offering long-lasting enzyme replacement with a potentially lower risk of immune reactions. By addressing the root cause of the disease enzyme deficiency, it helps clear harmful substances from cells, protect organ function, and improve quality of life.
While no therapy can cure Fabry disease, consistent treatment with Elfabrio can slow progression and support long-term well-being. Side effects are generally manageable under medical supervision, and regular monitoring ensures safety and effectiveness.
Elfabrio is a safe and effective therapy when prescribed and monitored by qualified healthcare providers. Patients are encouraged to stay informed, maintain regular appointments, and actively participate in their care plan.
References
- U.S. Food and Drug Administration (FDA). (2023). Elfabrio (pegunigalsidase alfa-iwxj) – Drug Approval Package.https://www.fda.gov/
- Chiesi Global Rare Diseases. (2024). Elfabrio (pegunigalsidase alfa) product information. https://www.chiesiglobalrarediseases.com/
- National Institutes of Health (NIH). (2024). Fabry disease: Genetics, treatment, and management. https://www.nih.gov/
- MedlinePlus. (2024). Fabry disease treatment overview. https://medlineplus.gov/
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: A Study to Learn About the Safety and Effects of the Study Drug PRX-102 in Children and Adolescents with Fabry Disease.
Summary: The aim of this study is to evaluate the safety and efficacy of pegunigalsidase alfa in Japanese patients (adults and adolescents) affected by Fabry disease. It is planned of a total of approximately 18-20 male and female Fabry disease patients between the ages of 13 and 60 years to be part of the study. The study is conducted in Japan.
Summary: A multi-centre, multi-country, observational, non-interventional, retrospective and prospective (hybrid) study among Fabry disease participants treated with pegunigalsidase alfa (Elfabrio®) in routine clinical care.
Related Latest Advances
Brand Information
- Hypersensitivity Reactions Including Anaphylaxis
- Infusion-Associated Reactions (IARs)
- Membranoproliferative Glomerulonephritis
2 “Infusion-associated reaction” includes nausea, vomiting, abdominal pain, diarrhea, fatigue, chills, malaise, non-cardiac chest pain, hypersensitivity, body temperature increased, burning sensation, neuralgia, agitation, throat irritation, pruritic rash, and flushing. Events occurring within 24 hours.
3 “Hypersensitivity” includes macular rash, pruritic rash, and face swelling. Events occurring within 24 hours.
4 The events of hypersensitivity and pruritic rash fall in both hypersensitivity and IAR categories.
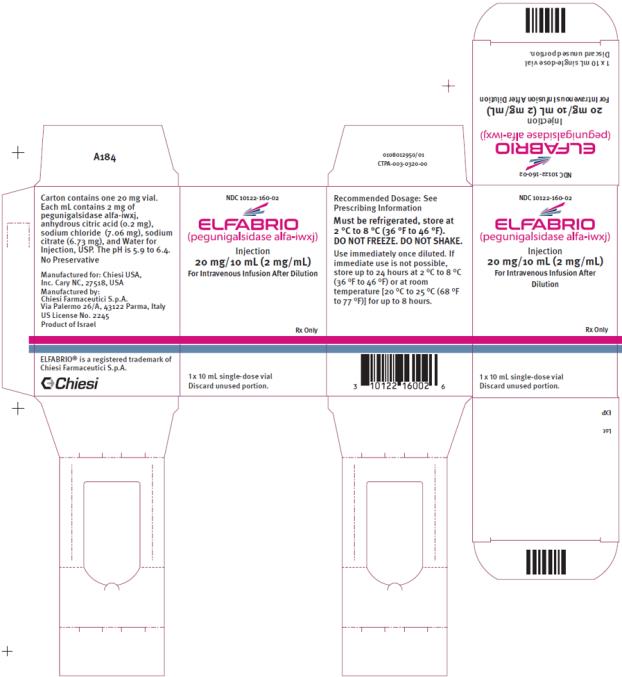
- One single-dose 20 mg/10 mLvial in a carton (NDC 10122-160-02)
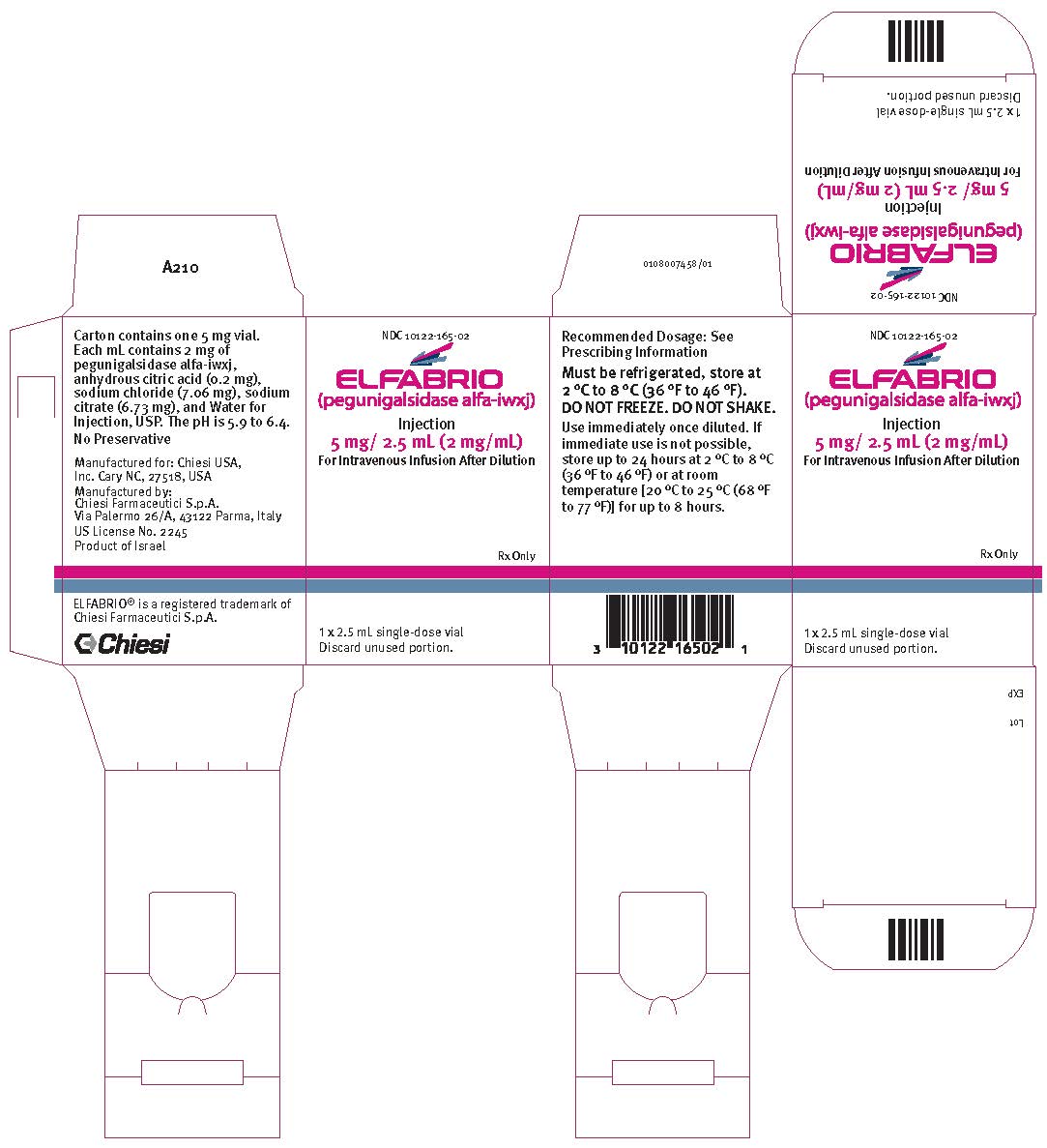
- One single-dose 5 mg/2.5 mL vial in a carton (NDC 10122-165-02)
- Five single-dose 20 mg/10 mL vials in a carton (NDC 10122-160-05)
- Ten single-dose 20 mg/10 mL vials in a carton (NDC 10122-160-10)