Brand Name
Pombiliti Atga
Generic Name
Cipaglucosidase Alfa-Atga
View Brand Information FDA approval date: October 11, 2023
Classification: Hydrolytic Lysosomal Glycogen-specific Enzyme
Form: Injection
What is Pombiliti Atga (Cipaglucosidase Alfa-Atga)?
POMBILITI is indicated, in combination with Opfolda, for the treatment of adult patients with late-onset Pompe disease (lysosomal acid alpha-glucosidase deficiency) weighing ≥40 kg and who are not improving on their current enzyme replacement therapy . POMBILITI is a hydrolytic lysosomal glycogen-specific enzyme indicated, in combination with Opfolda, an enzyme stabilizer, for the treatment of adult patients with late-onset Pompe disease (lysosomal acid alpha-glucosidase deficiency) weighing ≥40 kg and who are not improving on their current enzyme replacement therapy .
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Brand Information
POMBILITI (cipaglucosidase alfa-atga)
WARNING: SEVERE HYPERSENSITIVITY REACTIONS, INFUSION-ASSOCIATED REACTIONS, and RISK OF ACUTE CARDIORESPIRATORY FAILURE IN SUSCEPTIBLE PATIENTS
Hypersensitivity Reactions Including AnaphylaxisPatients treated with POMBILITI have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Appropriate medical support measures, including cardiopulmonary resuscitation equipment, should be readily available during POMBILITI administration. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, POMBILITI should be discontinued immediately, and appropriate medical treatment should be initiated. In patients with severe hypersensitivity reaction, desensitization measures to POMBILITI may be considered
Infusion-Associated Reactions (IARs)Patients treated with POMBILITI have experienced severe IARs. If severe IARs occur, immediately discontinue the POMBILITI infusion, initiate appropriate medical treatment, and assess the benefits and risks of readministering POMBILITI following severe IARs. Patients with an acute underlying illness at the time of POMBILITI infusion may be at greater risk for IARs. Patients with advanced Pompe disease may have compromised cardiac and respiratory function, which may predispose them to a higher risk of severe complications from IARs
Risk of Acute Cardiorespiratory Failure in Susceptible PatientsPatients susceptible to fluid volume overload, or those with acute underlying respiratory illness or compromised cardiac or respiratory function for whom fluid restriction is indicated may be at risk of serious exacerbation of their cardiac or respiratory status during POMBILITI infusion. More frequent monitoring of vitals should be performed during POMBILITI infusion in such patients
1INDICATIONS AND USAGE
POMBILITI is indicated, in combination with Opfolda, for the treatment of adult patients with late-onset Pompe disease (lysosomal acid alpha-glucosidase [GAA] deficiency) weighing ≥40 kg and who are not improving on their current enzyme replacement therapy (ERT).
2DOSAGE FORMS AND STRENGTHS
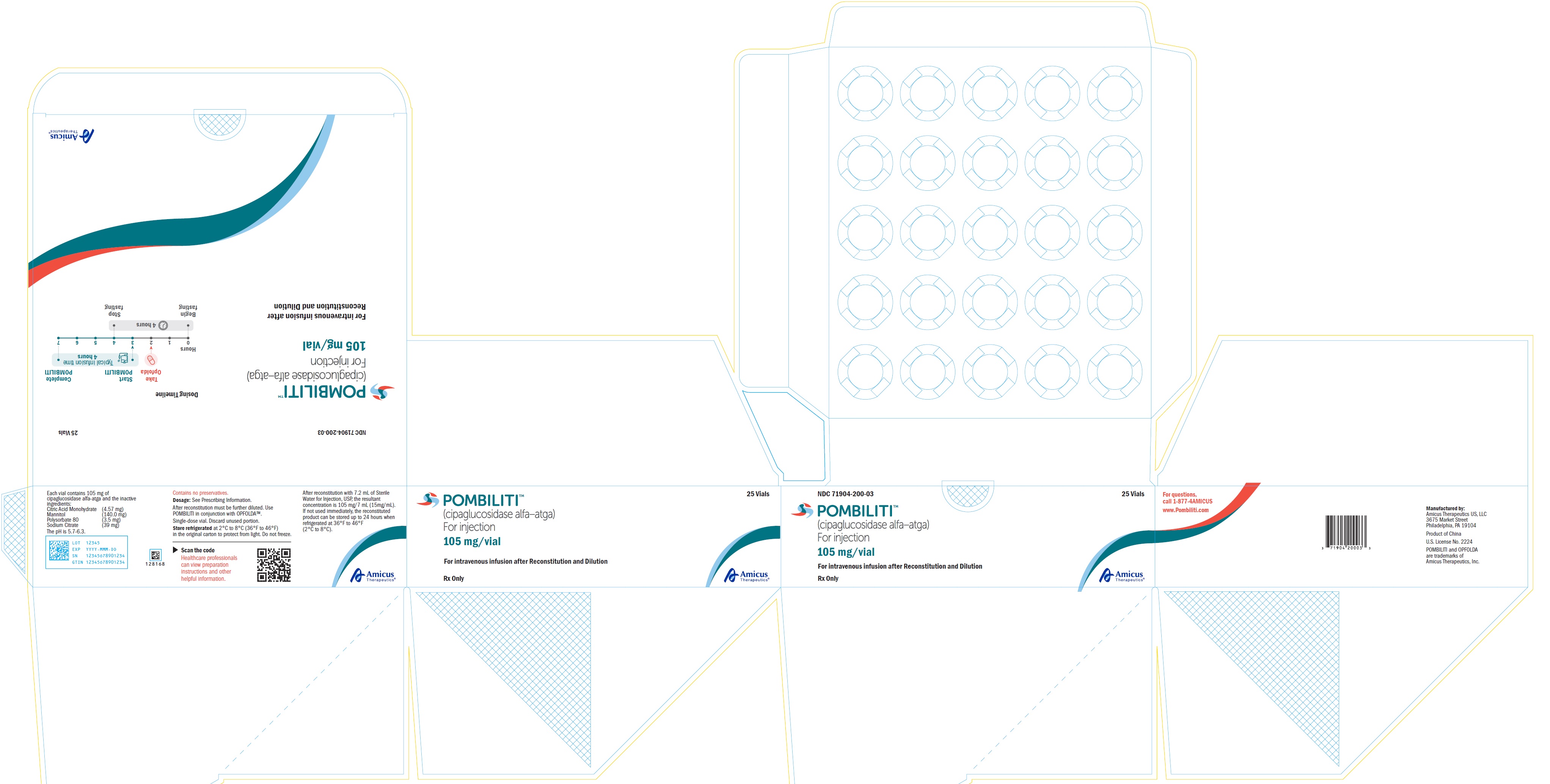
For injection: 105 mg of cipaglucosidase alfa-atga as a white to slightly yellowish lyophilized powder with a cake-like appearance in a single-dose vial for reconstitution
3CONTRAINDICATIONS
POMBILITI in combination with Opfolda is contraindicated in pregnancy
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions Including Anaphylaxis
- Infusion-Associated Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions from the Pooled Clinical Trials Including Trial 1
The pooled safety analysis from 3 clinical trials included 151 adult patients with late-onset Pompe disease (LOPD) treated with POMBILITI in combination with Opfolda including:
- 85 patients in the randomized, double-blind, active-controlled trial in adults (Trial 1)
- 37 patients in the open-label extension trial where patients switched from a non‑U.S.‑approved alglucosidase alfa product
- 29 patients in an open-label trial.
The total median duration of exposure in these trials was 21 months, with 120 patients having at least 12 months exposure to POMBILITI in combination with Opfolda. In these trials, 78% (n=117) of the patients received previous ERT (ERT‑experienced) with a mean treatment duration of 7.7 years.
In these trials, serious adverse reactions reported in 2 or more patients treated with POMBILITI in combination with Opfolda were anaphylaxis and urticaria. A total of 5 patients treated with POMBILITI in combination with Opfolda in these trials permanently discontinued POMBILITI due to adverse reactions, including 4 of these patients who discontinued the treatment because of a serious adverse reaction.
The most common adverse reactions (≥5%) reported in the pooled safety population of patients treated with POMBILITI in combination with Opfolda in the 3 clinical trials were headache, diarrhea, fatigue, nausea, abdominal pain and pyrexia.
In these trials, IARs were reported in 48 (32%) patients treated with POMBILITI in combination with Opfolda. IARs reported in more than 1 patient included headache, myalgia, diarrhea, nausea, fatigue, muscle spasms, pyrexia, dizziness, cough, chills, rash, vomiting, dyspnea, pain, abdominal distension, tachycardia, urticaria, flatulence, pruritus, abdominal pain, chest discomfort, flushing, hyperhidrosis, dysgeusia, hypotension, and hypertension
Adverse Reactions from Trial 1
Trial 1 (a randomized, double‑blind, active‑controlled trial) included 123 adult patients with LOPD who were randomized in a 2:1 ratio to receive treatment with POMBILITI in combination with Opfolda or a non-U.S.-approved alglucosidase alfa product with placebo
The duration of exposure was similar for both treatment groups (overall mean exposure of 12 months). Most patients (77%) were ERT‑experienced, and a majority of patients in both treatment groups had >5 years of prior treatment with ERT (69% and 63% of patients in the POMBILITI in combination with Opfolda group and the non-U.S.-approved alglucosidase alfa product with placebo group, respectively).
The most common adverse reactions (≥5%) reported in the patients who received POMBILITI in combination with Opfolda in Trial 1 were headache and diarrhea.
Table 2 summarizes frequent adverse reactions that occurred in patients treated with POMBILITI in combination with Opfolda in Trial 1. Trial 1 was not designed to demonstrate a statistically significant difference in the incidence of adverse reactions in the POMBILITI in combination with Opfolda and the non-U.S.-approved alglucosidase alfa product with placebo groups.
Table 2. Adverse Reactions that Occurred in Adults with LOPD at an Incidence of ≥2% in Trial 1
Additional adverse reactions reported in at least 2% of patients treated with POMBILITI in combination with Opfolda across the 3 clinical trials include: myalgia, arthralgia, increased blood pressure, pain, tremor, dyspepsia, asthenia, constipation, infusion site swelling, flank pain, malaise, paresthesia, somnolence, and decreased platelet count.
Immunogenicity: Anti-Drug Antibody-Associated Adverse Reactions in Trial 1
Anaphylaxis or serious infusion-associated reactions (IARs) occurred in 1 (1.5%) POMBILITI‑treated patient (who previously received U.S.-approved alglucosidase alfa or a non-U.S.-approved alglucosidase alfa product) who had peak anti-cipaglucosidase alfa-atga antibody titer of 6,553,600 during the 52-week treatment period
5DESCRIPTION
Cipaglucosidase alfa-atga is a hydrolytic lysosomal glycogen‑specific recombinant human α‑glucosidase (rhGAA) enzyme derived from a Chinese Hamster Ovary (CHO) cell line using perfusion methodology, resulting in cellularly (CHO)‑derived N‑glycans. Cipaglucosidase alfa‑atga is a glycoprotein with 1.3 mols of bis‑mannose‑6‑phosphate (bis‑M6P) per mol of enzyme. Cipaglucosidase alfa-atga has a molecular weight of approximately 110 kDa.
POMBILITI (cipaglucosidase alfa-atga) for injection is a sterile, white to slightly yellowish lyophilized powder with a cake-like appearance for intravenous use after reconstitution and dilution. Each single-dose vial contains 105 mg of cipaglucosidase alfa-atga and the inactive ingredients citric acid monohydrate (4.57 mg), mannitol (140 mg), polysorbate 80 (3.5 mg), and sodium citrate (39 mg). After reconstitution with Sterile Water for Injection, USP, the resultant concentration is 15 mg/mL with a pH of between 5.7 to 6.3.
6CLINICAL STUDIES
Trial 1 was a randomized, double‑blind, active‑controlled, international, multi‑center clinical trial (NCT#03729362) in patients ≥18 years old diagnosed with LOPD. Patients were randomized 2:1 to receive POMBILITI (20 mg/kg by intravenous infusion) in combination with Opfolda (260 mg orally for those ≥50 kg or 195 mg orally for those ≥40 kg to <50 kg) or a non-U.S.-approved alglucosidase alfa product with placebo every other week for 52 weeks. The efficacy population included a total of 123 patients of whom 95 (77%) had received prior treatment with U.S.-approved alglucosidase alfa or a non-U.S.-approved alglucosidase alfa product (ERT‑experienced) and 28 (23%) were ERT‑naïve. More than two thirds (n=64, 67%) of ERT‑experienced patients had been on ERT treatment for more than 5 years prior to entering Trial 1 (mean of 7.4 years).
Demographics, baseline sitting forced vital capacity (FVC) (% predicted), and 6-minute walk distance (6MWD) were generally similar between the 2 treatment groups (see
Key efficacy endpoints included assessment of sitting FVC (% predicted) and 6MWD (
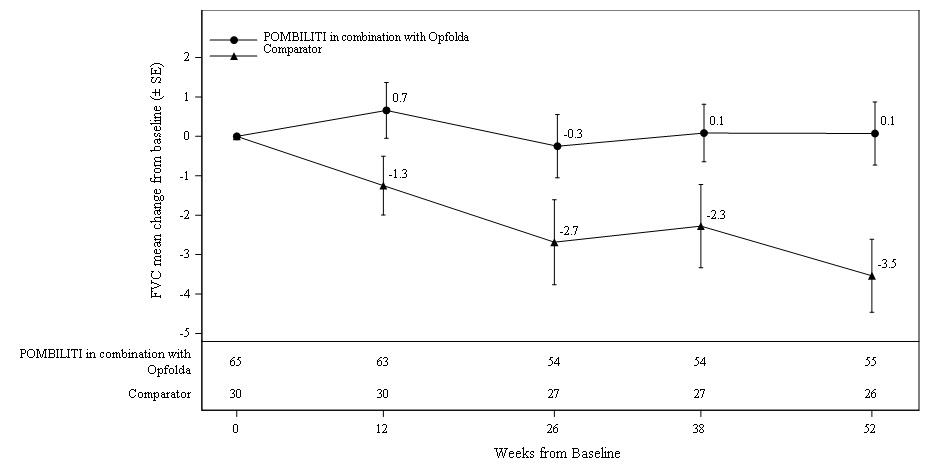
Sitting FVC (Percent‑predicted) at 52 Weeks
Patients treated with POMBILITI in combination with Opfolda showed a mean change in sitting FVC from baseline at Week 52 of -1.1% as compared with patients treated with a non-U.S.-approved alglucosidase alfa product with placebo of -3.3%; the estimated treatment difference was 2.3% (95% CI: 0.02, 4.62).
The ERT‑experienced patients treated with POMBILITI in combination with Opfolda showed a numerically favorable change in sitting FVC from baseline at Week 52 (
Table 5. Summary of Sitting FVC in Adults with LOPD by ERT Status at 52 Weeks in Trial 1
Figure 2. Mean Change (± SE) in Sitting FVC (% predicted) from Baseline to Week 52 in ERT-experienced Adults with LOPD in Trial 1
SE: standard error; FVC: forced vital capacity; ERT: enzyme replacement therapy; LOPD: late-onset Pompe disease
∗ A U.S.-approved alglucosidase alfa product was not used in this clinical trial. Conclusions cannot be drawn from this clinical trial regarding comparative effectiveness between a U.S.-approved alglucosidase alfa product and POMBILITI in combination with Opfolda for the treatment of adult patients with LOPD weighing ≥40 kg and who are not improving on their current ERT.
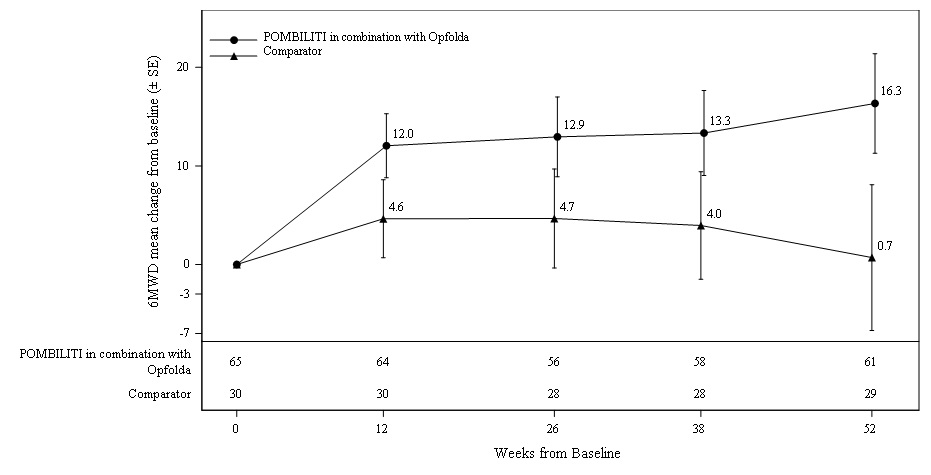
6 Minute Walk Distance (6MWD) at 52 Weeks
Patients treated with POMBILITI in combination with Opfolda walked on average 21 meters farther from baseline as compared to those treated with a non-U.S.-approved alglucosidase alfa product with placebo who walked 8 meters farther from baseline; the estimated treatment difference was 14 meters (95% CI: -1, 28).
The ERT‑experienced patients treated with POMBILITI in combination with Opfolda showed a numerically favorable change in 6MWD from baseline at Week 52 (
Table 6. Summary of 6MWD in Adults with LOPD by ERT Status at 52 Weeks in Trial 1
Figure 3. Mean Change (± SE) of 6MWD from Baseline to Week 52 in ERT‑experienced Adults with LOPD in Trial 1
SE: standard error; 6MWD: 6-minute walk distance; ERT: enzyme replacement therapy; LOPD: late-onset Pompe disease
∗ A U.S.-approved alglucosidase alfa product was not used in this clinical trial. Conclusions cannot be drawn from this clinical trial regarding comparative effectiveness between a U.S.-approved alglucosidase alfa product and POMBILITI in combination with Opfolda for the treatment of adult patients with LOPD weighing ≥40 kg and who are not improving on their current ERT.
7HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
POMBILITI (cipaglucosidase alfa-atga) for injection is supplied as a sterile, white to slightly yellowish lyophilized powder with a cake-like appearance in a single‑dose vial. POMBILITI does not contain any preservatives. See
Table 7. POMBILITI Cartons
Storage and Handling
Store refrigerated at 2℃ to 8℃ (36℉ to 46℉) in the original carton to protect from light. Do not freeze.
For storage of the reconstituted solution and diluted solution
8PATIENT COUNSELING INFORMATION
POMBILITI must be administered in combination with Opfolda. Refer to the Opfolda Prescribing Information for Opfolda patient counseling information.
Administration
Advise the patient and caregiver to follow the timeline recommendations for taking Opfolda prior to the intravenous infusion with POMBILITI [see .
Advise the patient and caregiver to follow the timeline recommendations for taking Opfolda prior to the intravenous infusion with POMBILITI [see .
Hypersensitivity Reactions (Including Anaphylaxis) and Infusion-Associated Reactions (IARs)
Advise the patient and caregiver that reactions related to the infusion may occur during and after POMBILITI in combination with Opfolda treatment, including life-threatening hypersensitivity reactions, including anaphylaxis, and IARs. Inform the patient and caregiver of the signs and symptoms of hypersensitivity reactions and IARs and to seek medical care should signs and symptoms occur [see .
Advise the patient and caregiver that reactions related to the infusion may occur during and after POMBILITI in combination with Opfolda treatment, including life-threatening hypersensitivity reactions, including anaphylaxis, and IARs. Inform the patient and caregiver of the signs and symptoms of hypersensitivity reactions and IARs and to seek medical care should signs and symptoms occur [see .
Risk of Acute Cardiorespiratory Failure
Advise the patient and caregiver that a patient with underlying respiratory illness or compromised cardiac or respiratory function may be at risk of acute cardiorespiratory failure from volume overload during POMBILITI infusion and to seek medical care should signs and symptoms occur [see .
Advise the patient and caregiver that a patient with underlying respiratory illness or compromised cardiac or respiratory function may be at risk of acute cardiorespiratory failure from volume overload during POMBILITI infusion and to seek medical care should signs and symptoms occur [see .
Embryo-Fetal Toxicity
POMBILITI in combination with Opfolda may cause embryo-fetal harm. Advise a female patient and caregiver to inform their healthcare provider of a known or suspected pregnancy [see .
POMBILITI in combination with Opfolda may cause embryo-fetal harm. Advise a female patient and caregiver to inform their healthcare provider of a known or suspected pregnancy [see .
Advise a female of reproductive potential to use effective contraception during treatment with POMBILITI in combination with Opfolda and for at least 60 days after the last dose
Lactation
Advise a lactating female not to breastfeed during treatment with POMBILITI in combination with Opfolda [see .
Advise a lactating female not to breastfeed during treatment with POMBILITI in combination with Opfolda [see .
Infertility
Advise the male or female of reproductive potential that POMBILITI in combination with Opfolda may impair fertility [see .
Advise the male or female of reproductive potential that POMBILITI in combination with Opfolda may impair fertility [see .
Manufactured by:
U.S. License No. 2224
POMBILITI and Opfolda are registered trademarks of Amicus Therapeutics, Inc.
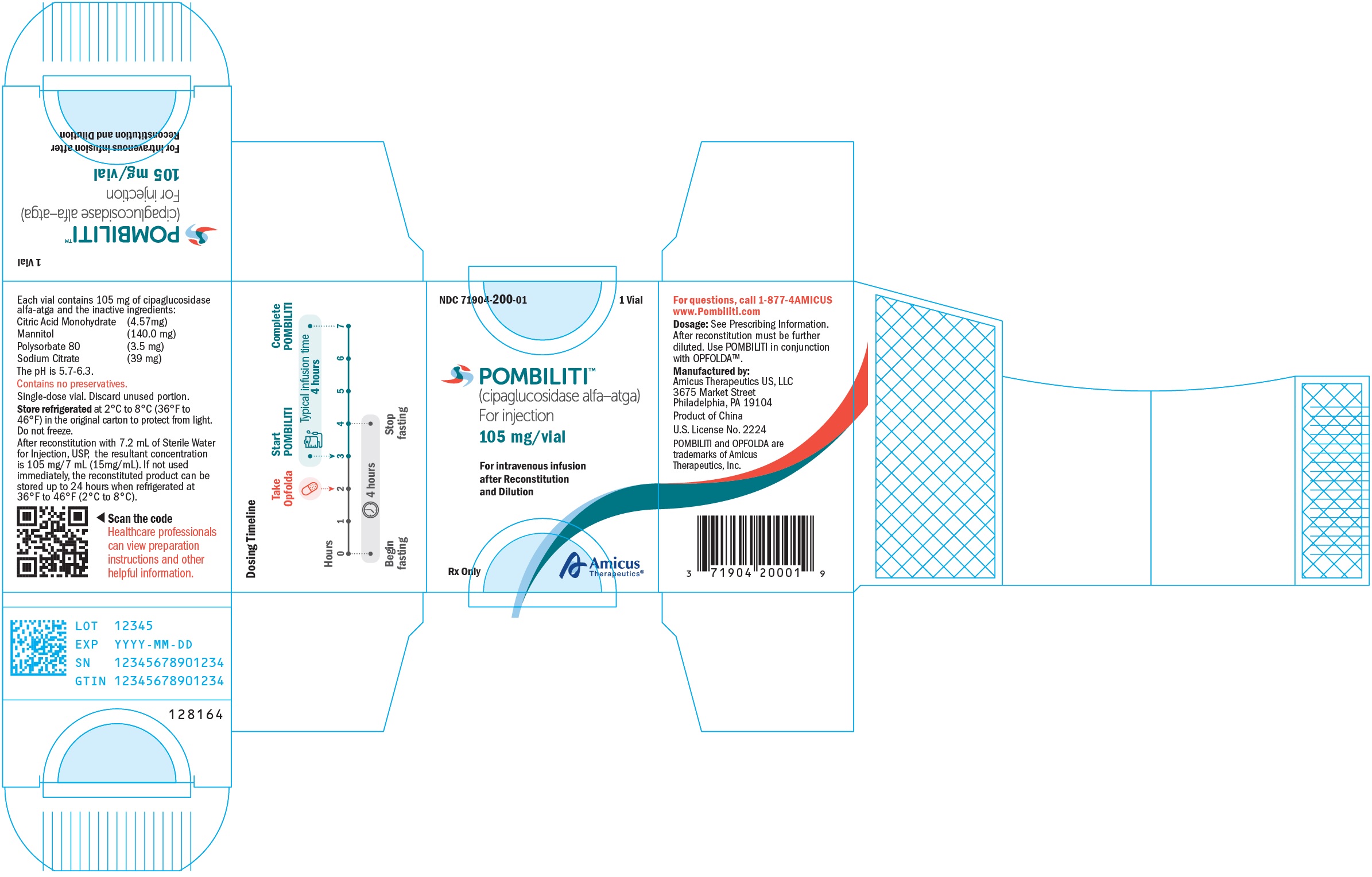
9PRINCIPAL DISPLAY PANEL - NDC: 71904-200-01 - 105 mg/vial 1 Count Vial Label
10PRINCIPAL DISPLAY PANEL - NDC: 71904-200-01 - 105 mg/vial 1 Count Carton Label
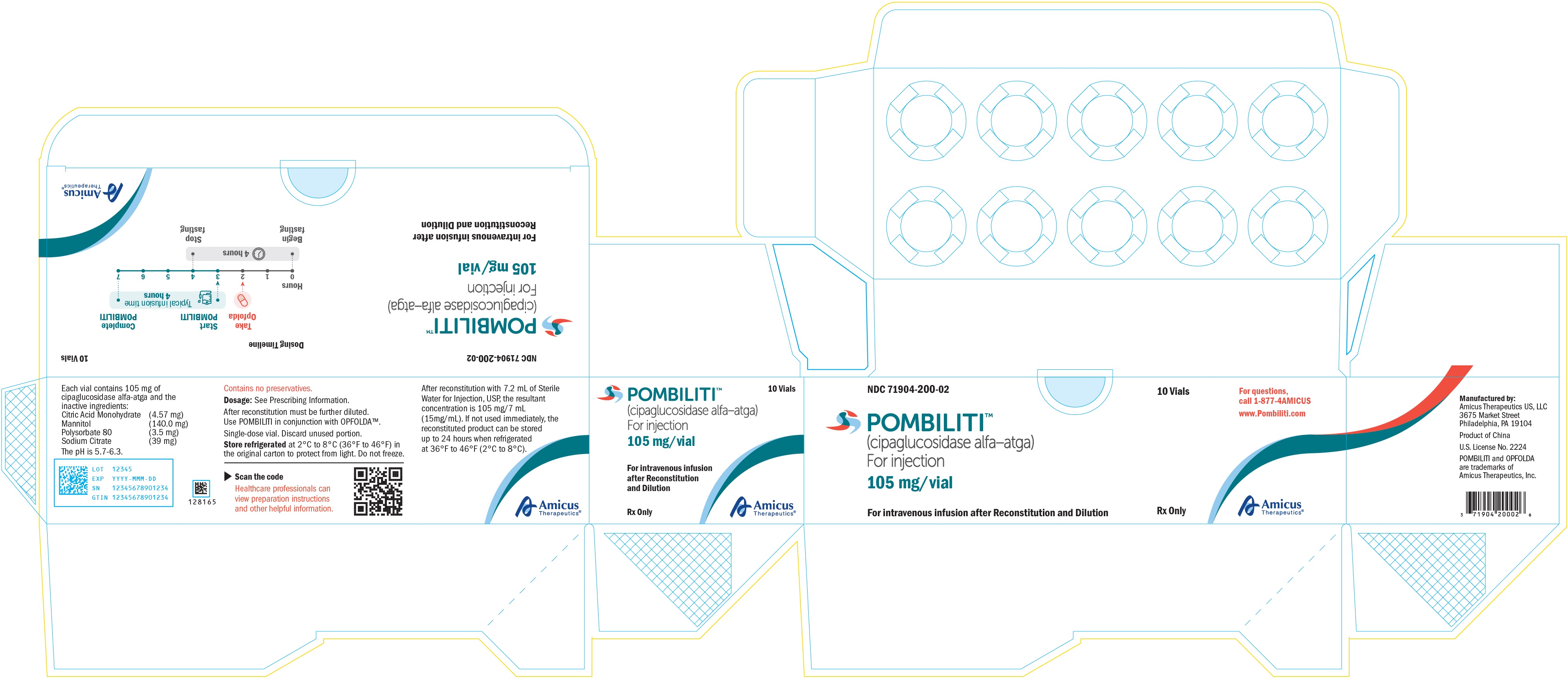
11PRINCIPAL DISPLAY PANEL - NDC: 71904-200-02 - 105 mg/vial 10 Count Multipack Label
12PRINCIPAL DISPLAY PANEL - NDC: 71904-200-03 - 105 mg/vial 25 Count Multipack Label