Brand Name
Cytomel
Generic Name
Liothyronine
View Brand Information FDA approval date: May 08, 1956
Classification: l-Triiodothyronine
Form: Injection, Tablet
What is Cytomel (Liothyronine)?
Liothyronine sodium injection is indicated in the treatment of myxedema coma/precoma. Liothyronine sodium injection can be used in patients allergic to desiccated thyroid or thyroid extract derived from pork or beef.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Cytomel (liothyronine sodium)
WARNING: NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS
- Thyroid hormones, including CYTOMEL, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss.
- In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction.
- Larger doses may produce serious or even life-threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects
1DOSAGE FORMS AND STRENGTHS
Tablets (round, white to off-white) available as follows:
- 5 mcg: debossed with KPI on one side and 115 on the other side
- 25 mcg: scored on one side and debossed with KPI and 116 on the other side
- 50 mcg: scored on one side and debossed with KPI and 117 on the other side
2CONTRAINDICATIONS
CYTOMEL is contraindicated in patients with uncorrected adrenal insufficiency
3ADVERSE REACTIONS
Adverse reactions associated with CYTOMEL therapy are primarily those of hyperthyroidism due to therapeutic overdosage
General: fatigue, increased appetite, weight loss, heat intolerance, fever, excessive sweating
Central nervous system: headache, hyperactivity, nervousness, anxiety, irritability, emotional lability, insomnia
Musculoskeletal: tremors, muscle weakness and cramps
Cardiovascular: palpitations, tachycardia, arrhythmias, increased pulse and blood pressure, heart failure, angina, myocardial infarction, cardiac arrest
Respiratory: dyspnea
Gastrointestinal: diarrhea, vomiting, abdominal cramps, elevations in liver function tests
Dermatologic: hair loss, flushing
Endocrine: decreased bone mineral density
Reproductive: menstrual irregularities, impaired fertility
4OVERDOSAGE
The signs and symptoms of overdosage are those of hyperthyroidism
Reduce the CYTOMEL dose or temporarily discontinued if signs or symptoms of overdosage occur. Initiate appropriate supportive treatment as dictated by the patient's medical status.
For current information on the management of poisoning or overdosage, contact the National Poison Control Center at 1-800-222-1222 or
5DESCRIPTION
CYTOMEL tablets contain the active ingredient, liothyronine (L-triiodothyronine or LT
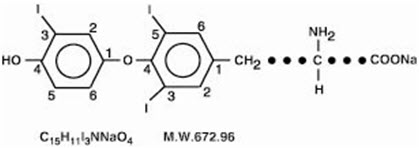
CYTOMEL tablets contain liothyronine sodium equivalent to liothyronine in 5 mcg, 25 mcg, and 50 mcg. Inactive ingredients consist of calcium sulfate, corn starch, gelatin, stearic acid, sucrose and talc.
6HOW SUPPLIED/STORAGE AND HANDLING
CYTOMEL tablets (round, white to off-white) are supplied as follows:
7PRINCIPAL DISPLAY PANEL - 5 mcg Tablet Bottle Label
NDC 60793-115-01
Pfizer
CYTOMEL®
liothyronine sodium tablets
liothyronine sodium tablets
5 mcg
100 Tablets

8PRINCIPAL DISPLAY PANEL - 25 mcg Tablet Bottle Label
NDC 60793-116-01
Pfizer
CYTOMEL®
liothyronine sodium tablets
liothyronine sodium tablets
25 mcg
100 Tablets

9PRINCIPAL DISPLAY PANEL - 50 mcg Tablet Bottle Label
NDC 60793-117-01
Pfizer
CYTOMEL®
liothyronine sodium tablets
liothyronine sodium tablets
50 mcg
100 Tablets
