Tecvayli
What is Tecvayli (Teclistamab)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of this study is to describe the use of teclistamab/talquetamab in the treatment of patients with RRMM outside of clinical trials.
Summary: The purpose of this study is to assess the safety of teclistamab in in routine clinical practice when given as monotherapy in Indian participants with relapsed and refractory multiple myeloma (RRMM) (that is, a blood cancer that comes back after treatment or does not respond to treatment) who have previously received at least 3 prior lines of therapy including an immunomodulatory agent, a proteaso...
Summary: The purpose of this study is to compare the effectiveness of either talquetamab plus pomalidomide (Tal-P) or talquetamab plus teclistamab (Tal-Tec) with elotuzumab, pomalidomide, and dexamethasone (EPd) or pomalidomide, bortezomib, and dexamethasone (PVd).
Related Latest Advances
Brand Information
- Cytokine Release Syndrome
- Neurologic Toxicity including ICANS
- Hepatotoxicity
- Infections
- Neutropenia
- Hypersensitivity and Other Administration Reactions
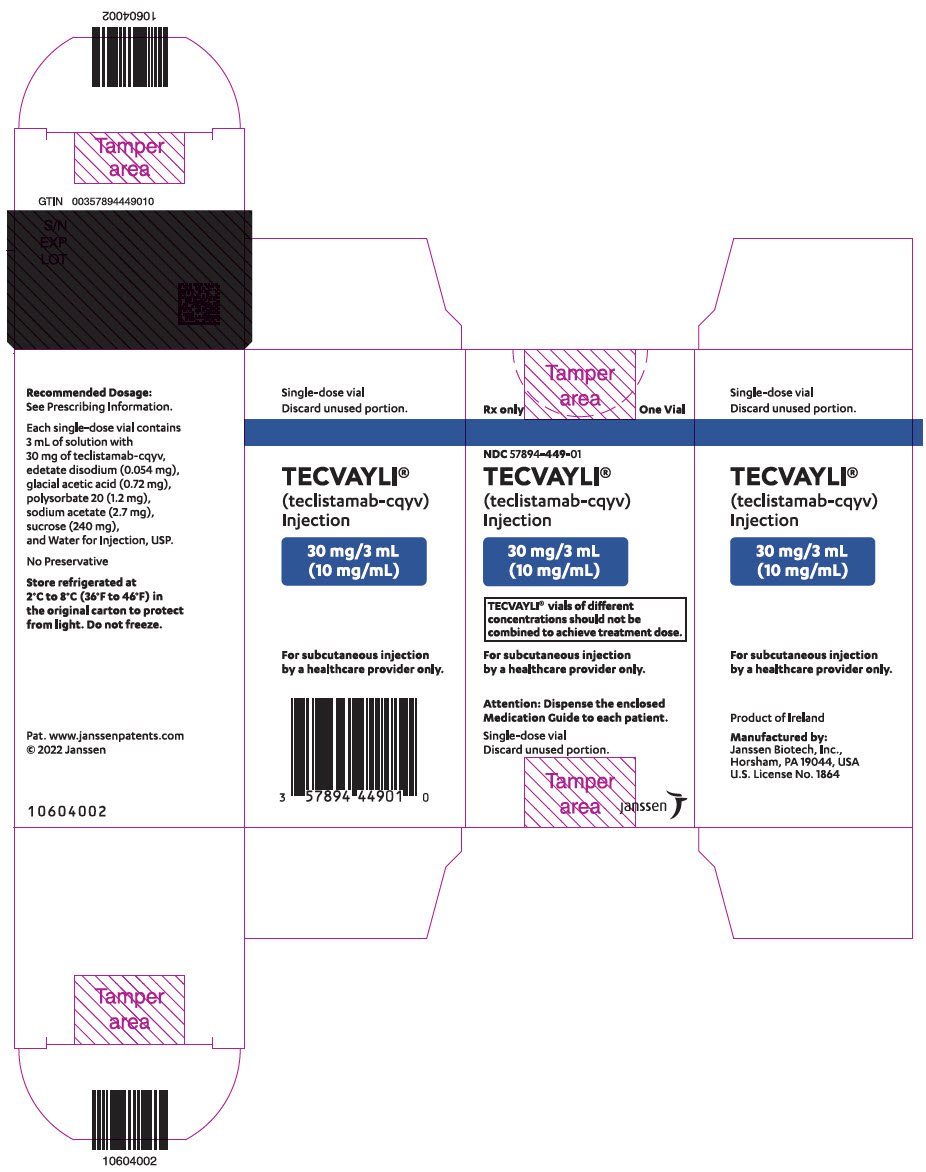
- One 30 mg/3 mL (10 mg/mL) single-dose vial in a carton: NDC: 57894-449-01
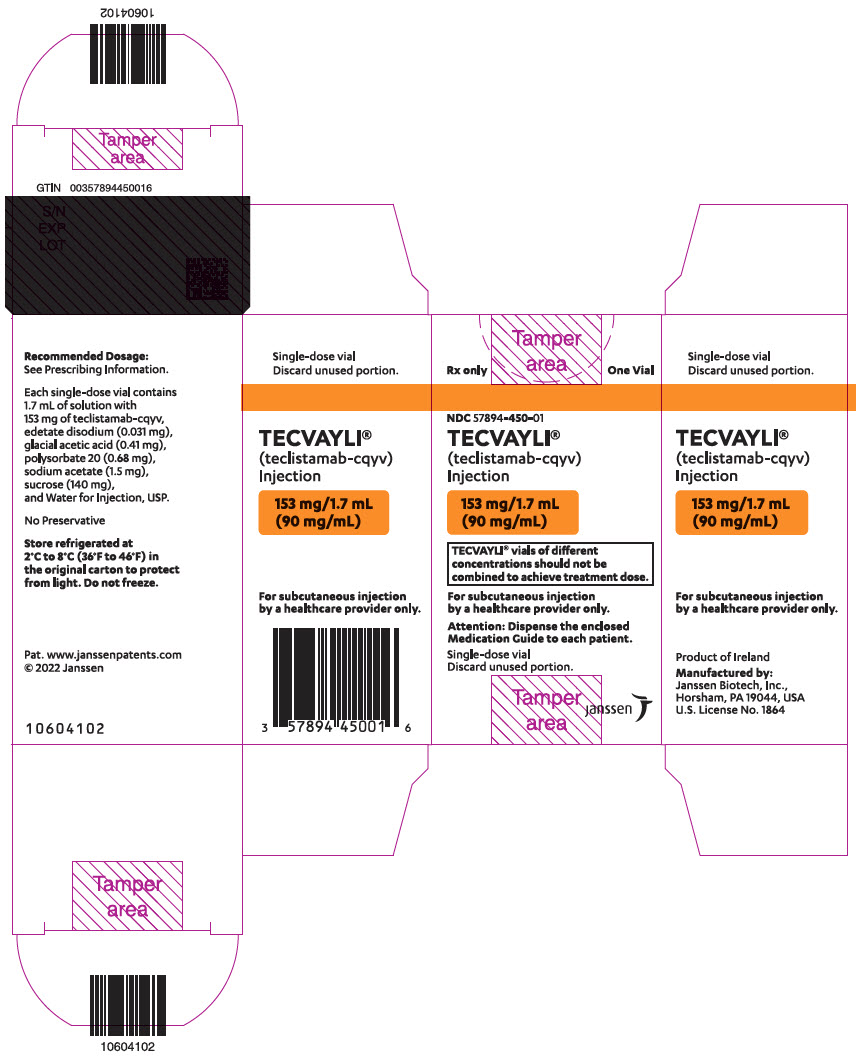
- One 153 mg/1.7 mL (90 mg/mL) single-dose vial in a carton: NDC: 57894-450-01