Talzenna
What is Talzenna (Talazoparib)?
Approved To Treat
Related Clinical Trials
Summary: This phase II trial tests whether ZEN003694 (ZEN-3694) in combination with talazoparib works to shrink tumors in patients with solid tumors that are unlikely to be cured or controlled with treatment and that may have spread from where it first started to nearby tissue, lymph nodes, or distant parts of the body (advanced). Another aim of this study is to find out if, and how, patients' genes influe...
Summary: The main objectif is to determine the efficacy of a maintenance treatment combining Talazoparib and Avelumab after platinum-based chemotherapy in patients with locally advanced/metastatic urothelial carcinoma.
Summary: This phase Ib trial is to find out the best dose, possible benefits and/or side effects of talazoparib when given in combination with palbociclib, axitinib, or crizotinib in treating patients with solid tumors that has spread to nearby tissue or lymph nodes (locally advanced) or other places in the body (metastatic). PARPs are proteins that help repair damaged DNA, the genetic material that serves...
Related Latest Advances
Brand Information
- Myelodysplastic Syndrome/Acute Myeloid Leukemia
- Myelosuppression
- 0.1 mg hard hypromellose (HPMC) capsule that contains 0.145 mg talazoparib tosylate equivalent to 0.1 mg talazoparib free base, or
- 0.25 mg HPMC capsule that contains 0.363 mg talazoparib tosylate equivalent to 0.25 mg talazoparib free base, or
- 0.35 mg HPMC capsule that contains 0.509 mg talazoparib tosylate equivalent to 0.35 mg talazoparib free base, or
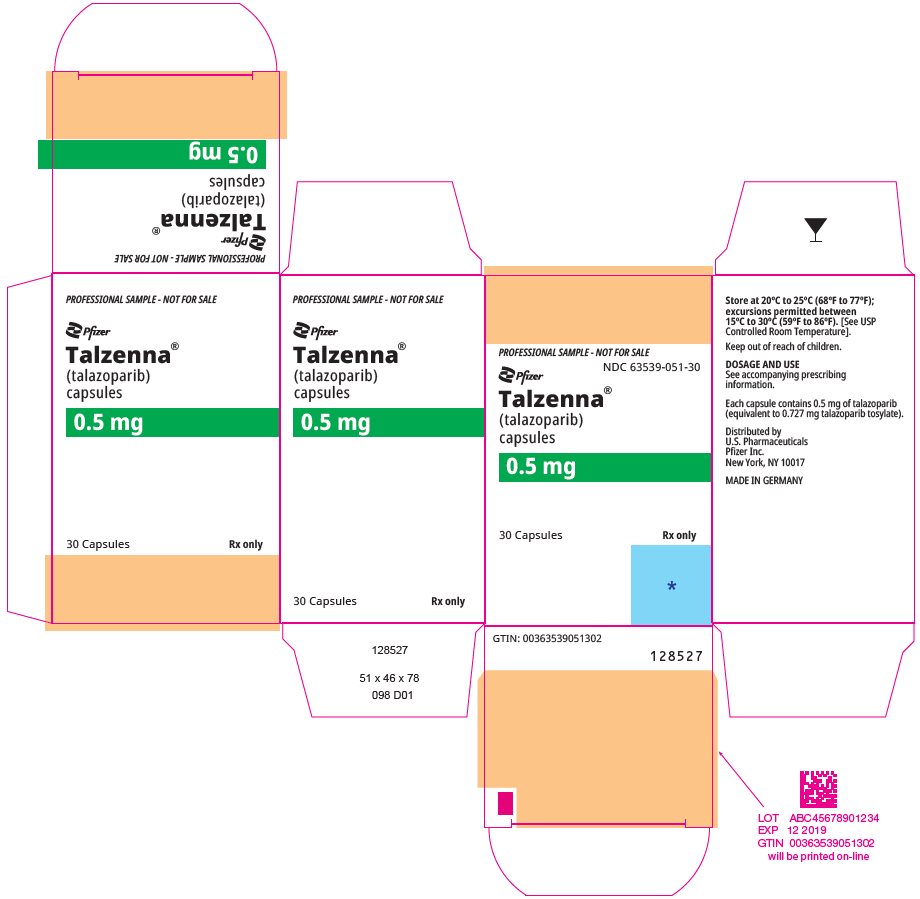
- 0.5 mg HPMC capsule that contains 0.727 mg talazoparib tosylate equivalent to 0.5 mg talazoparib free base, or
- 0.75 mg HPMC capsule that contains 1.09 mg talazoparib tosylate equivalent to 0.75 mg talazoparib free base, or
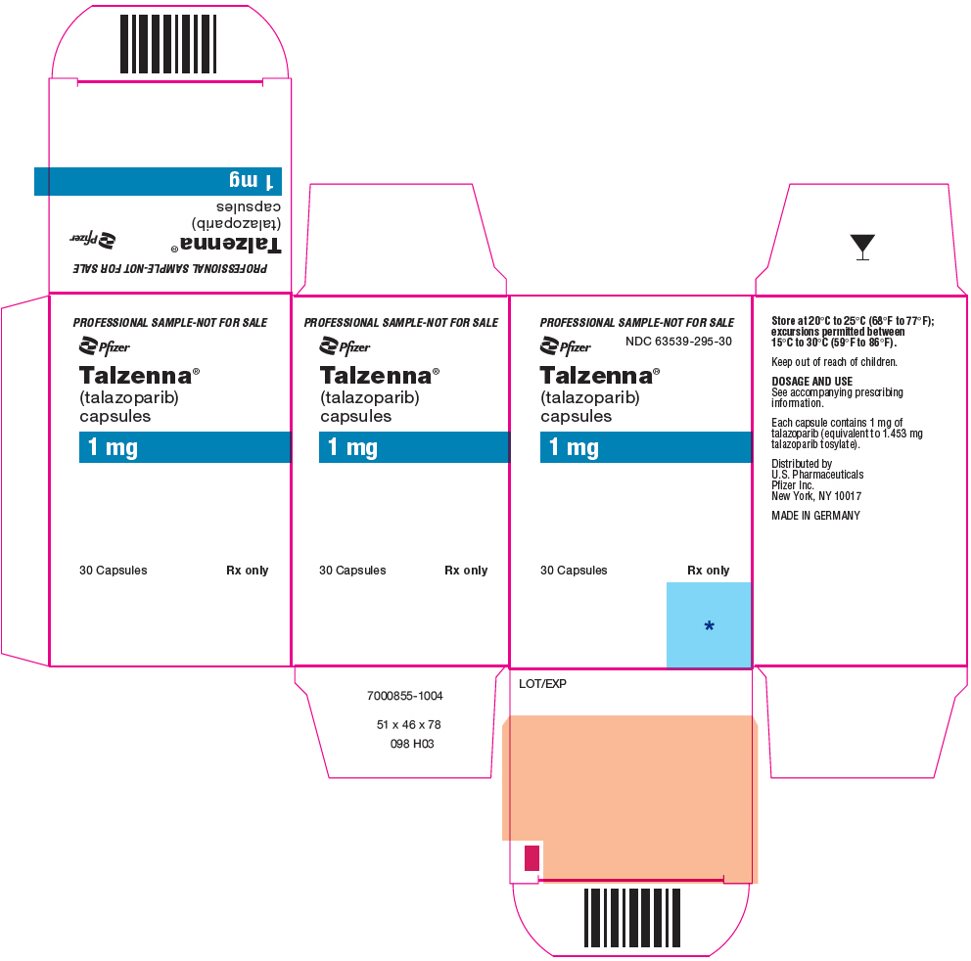
- 1 mg HPMC capsule that contains 1.453 mg talazoparib tosylate equivalent to 1 mg talazoparib free base.
- MDS/AML: Advise patients to contact their healthcare provider if they experience weakness, feeling tired, fever, weight loss, frequent infections, bruising, bleeding easily, breathlessness, blood in urine or stool, and/or laboratory findings of low blood cell counts, or a need for blood transfusions. This may be a sign of hematological toxicity or a more serious uncommon bone marrow problem called MDS or AML, which have been reported in patients who received PARP inhibitors [see .
- Myelosuppression: Advise patients that TALZENNA may affect hematopoiesis and can cause anemia, leukopenia/neutropenia, and/or thrombocytopenia [see .
- Administration Instructions: Advise patients that TALZENNA can be taken once daily with or without food. Instruct patients that if they miss a dose of TALZENNA, they should take their next normal dose at the usual time. Also advise patients to swallow each capsule whole, and that capsules must not be opened or dissolved [see .
- Embryo-Fetal Toxicity: Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the risk to a fetus and potential loss of the pregnancy [see . Advise females of reproductive potential to use effective contraception during treatment with TALZENNA and for 7 months after the last dose. Advise male patients with female partners of reproductive potential or who are pregnant to use effective contraception during treatment and for 4 months after receiving the last dose of TALZENNA [see .
- Lactation: Advise patients not to breastfeed while taking TALZENNA and for 1 month after receiving the last dose [see .