Brand Name
Imcivree
Generic Name
Setmelanotide
View Brand Information FDA approval date: December 14, 2020
Classification: Melanocortin 4 Receptor Agonist
Form: Solution
What is Imcivree (Setmelanotide)?
IMCIVREE is indicated to reduce excess body weight and maintain weight reduction long term in adults and pediatric patients aged 2 years and older with syndromic or monogenic obesity due to: Bardet-Biedl syndrome [see Dosage and Administration.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Phase 3, Double Blind, Randomized, Placebo-Controlled Trial to Evaluate the Efficacy and Safety of Setmelanotide in Patients With Acquired Hypothalamic Obesity
Summary: This is a sub-study of Study RM-493-040 (NCT05774756). The goal of this sub-study is to learn how well Setmelanotide works to improve weight reduction, hunger, and quality of life in patients 4 years of age and older with congenital Hypothalamic Obesity (cHO). To determine how well setmelanotide works and how safe it is, patients with cHO will take a daily injection of either setmelanotide or plac...
A Phase 2 Open-label Study of Setmelanotide in Patients With Prader-Willi Syndrome
Summary: This is a Phase 2, open-label study designed to assess the safety and efficacy of setmelanotide in patients with obesity due to Prader-Willi Syndrome (PWS) who are 6 to 65 years of age. Up to 20 patients are planned to be enrolled. Patients will take a daily subcutaneous dose of open-label setmelanotide for up to 52 weeks.
Related Latest Advances
Brand Information
Imcivree (setmelanotide)
1INDICATIONS AND USAGE
IMCIVREE is indicated to reduce excess body weight and maintain weight reduction long term in adults and pediatric patients aged 2 years and older with syndromic or monogenic obesity due to:
- Bardet-Biedl syndrome (BBS)
- Pro-opiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency as determined by an FDA-approved test demonstrating variants in
Limitations of Use:
IMCIVREE is
- Obesity due to suspected POMC, PCSK1, or LEPR deficiency with
- Other types of obesity not related to BBS or POMC, PCSK1, or LEPR deficiency, including obesity associated with other genetic syndromes and general (polygenic) obesity
2DOSAGE FORMS AND STRENGTHS
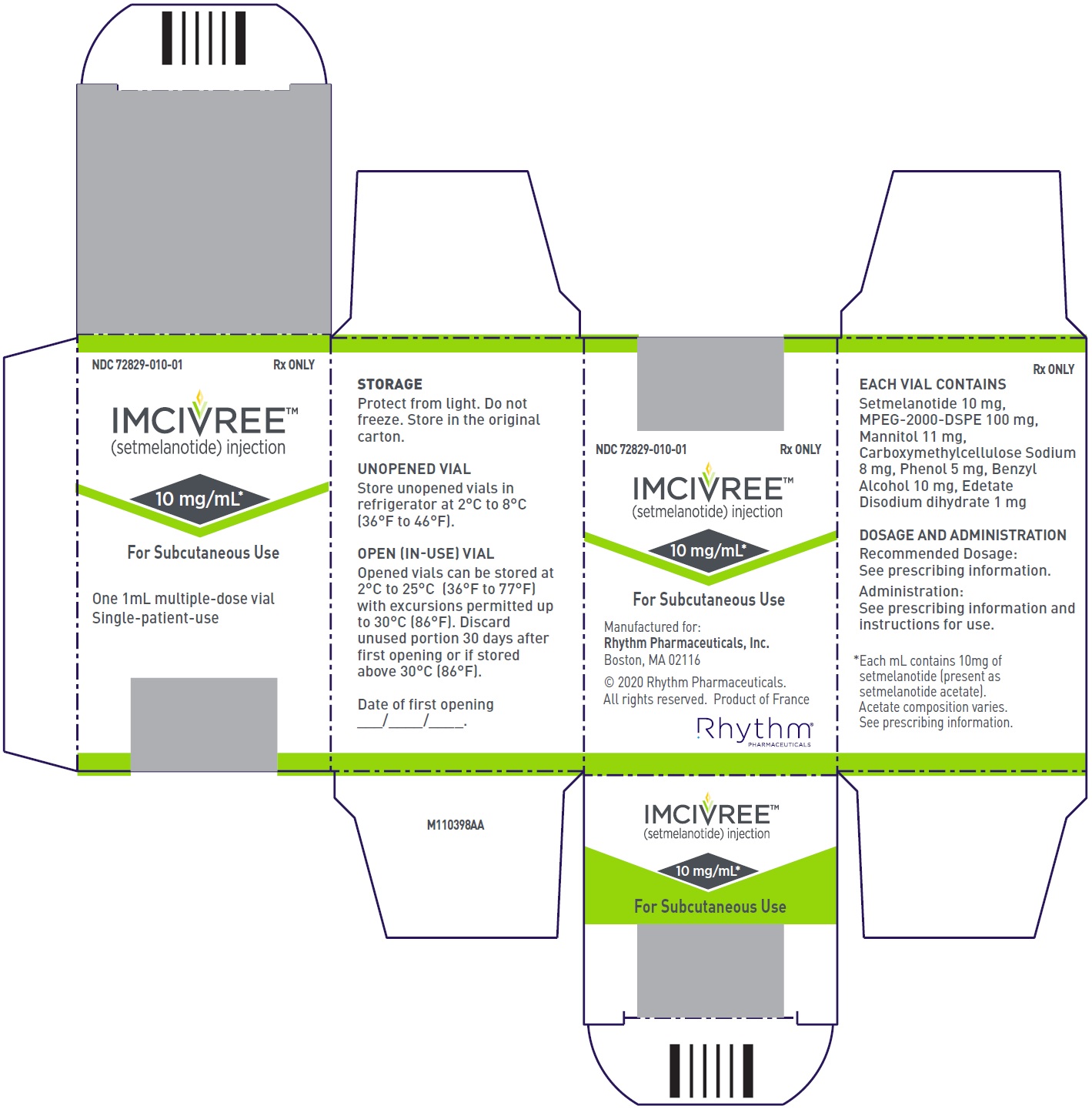
Injection: 10 mg/mL, clear to slightly opalescent, colorless to slightly yellow solution in a 1-mL multiple-dose vial for single patient use.
3CONTRAINDICATIONS
IMCIVREE is contraindicated in patients with a prior serious hypersensitivity reaction to setmelanotide or any of the excipients in IMCIVREE. Serious hypersensitivity reactions have included anaphylaxis
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Disturbance in Sexual Arousal
- Depression and Suicidal Ideation
- Hypersensitivity Reactions
- Skin Hyperpigmentation, Darkening of Pre-Existing Nevi, and Development of New Melanocytic Nevi
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Bardet-Biedl Syndrome (Adults and Pediatric Patients Aged 6 Years and Older)
The safety of IMCIVREE was evaluated in a clinical study, which included a 14 week, randomized, double-blind, placebo-controlled period followed by a 52-week open-label, treatment period, in 44 patients aged 6 years and older with obesity and a clinical diagnosis of BBS (Study 1)
During the 14-week placebo-controlled period in Study 1, the most common reported adverse reactions in IMCIVREE-treated patients when compared to placebo-treated patients were hyperpigmentation disorders (67% vs 0%, respectively) and vomiting (11% vs 0%, respectively).
Adverse reactions were also evaluated during the 52-week active-treatment period, defined as the period from randomization to Week 52 in patients initially randomized to IMCIVREE, and from Week 14 to Week 66 in patients initially randomized to placebo.
POMC, PCSK1, and LEPR Deficiency (Adults and Pediatric Patients Aged 6 Years and Older)
The safety of IMCIVREE was evaluated in two 52-week, open-label clinical studies of 27 patients aged 6 years and older with obesity due to POMC, PCSK1, or LEPR deficiency with POMC, PCSK1, or LEPR genes that are interpreted as pathogenic, likely pathogenic, or of uncertain significance (Study 2 and Study 3)
Table 4summarizes the adverse reactions that occurred in the open-label studies during the first 52 weeks of treatment in 3 or more patients treated with IMCIVREE.
POMC, PCSK1, and LEPR Deficiency and BBS (Pediatric Patients Aged 2 to <6 Years)
The safety of IMCIVREE was evaluated in one 52-week, open-label clinical study of 12 patients aged 2 to less than 6 years with obesity due to POMC, PCSK1, or LEPR deficiency with
Table 5summarizes the adverse reactions that occurred in the open-label study during 52 weeks of treatment in 3 or more patients treated with IMCIVREE.
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of IMCIVREE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Hypersensitivity, including anaphylaxis
5OVERDOSAGE
In the event of an overdose initiate appropriate supportive treatment according to the patient’s clinical signs and symptoms.
6DESCRIPTION
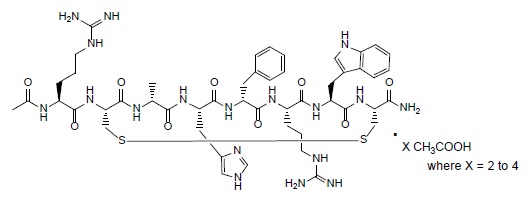
IMCIVREE contains setmelanotide acetate, a melanocortin 4 (MC4) receptor agonist. Setmelanotide is an 8 amino acid cyclic peptide analog of endogenous melanocortin peptide α-MSH (alpha-melanocyte stimulating hormone).
The chemical name for setmelanotide acetate is acetyl-L-arginyl-L-cysteinyl-D-alanyl-L-histidinyl-D-phenylalanyl-L-arginyl-L-tryptophanyl-L-cysteinamide cyclic (2→8)-disulfide acetate. Its molecular formula is C
The chemical structure of setmelanotide acetate is:
IMCIVREE (setmelanotide) injection is a sterile clear to slightly opalescent, colorless to slightly yellow solution for subcutaneous use. Each 1 mL single-patient use vial of IMCIVREE contains 10 mg of setmelanotide provided as setmelanotide acetate, which is a salt with 2 to 4 molar equivalents of acetate, and the following inactive ingredients: 10 mg benzyl alcohol, 8 mg carboxymethylcellulose sodium (average MWt 90,500), 1 mg edetate disodium dihydrate, 100 mg N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl- glycero-3- phosphoethanolamine sodium salt, 11 mg mannitol, 5 mg phenol, hydrochloric acid, sodium hydroxide and Water for Injection. The pH of IMCIVREE is 5 to 6.
7HOW SUPPLIED/STORAGE AND HANDLING
IMCIVREE injection is supplied as:
- 10 mg/mL, clear to slightly opalescent, colorless to slightly yellow solution in a 1-mL multiple-dose vial for single patient use
- Package of 1 multiple-dose vial: NDC 72829-010-01
Store unopened IMCIVREE vials in the refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton. After removal from the refrigerator, vials may be kept at temperatures ranging from refrigerated to room temperature (2°C to 25°C [36°F to 77°F]) for up to 30 days with brief excursions up to 30°C (86°F). After the vial is punctured (opened), discard after 30 days. See
Table 14 Recommended Storage for IMCIVREE Vials
8PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Disturbance in Sexual Arousal
Inform patients that sexual adverse reactions, including spontaneous erection, may occur in patients treated with IMCIVREE. Advise patients to seek emergency medical treatment if an erection lasts longer than 4 hours
Depression and Suicidal Ideation
Inform patients or caregivers that IMCIVREE may cause depression or suicidal ideation. Advise patients or caregivers to report any new or worsening symptoms of depression, suicidal thoughts or behaviors, or unusual changes in mood or behavior
Hypersensitivity Reactions
Inform patients that serious hypersensitivity reactions have been reported with use of IMCIVREE. Advise patients on the symptoms of hypersensitivity reactions and instruct them to stop taking IMCIVREE and seek medical advice promptly if such symptoms occur
Skin Hyperpigmentation, Darkening of Pre-Existing Nevi, and Development of New Melanocytic Nevi
Inform patients or caregivers that skin darkening occurs in the majority of patients treated with IMCIVREE because of its mechanism of action. This change is reversable upon discontinuation of IMCIVREE. Inform patients and caregivers that the development of new melanocytic nevi may occur. Inform patients or caregivers that they should have a full body skin examination before starting and during treatment with IMCIVREE to monitor these changes
Pregnancy
Advise patients who may become pregnant to inform their healthcare provider of a known or suspected pregnancy
Lactation
Advise patients that treatment with IMCIVREE is not recommended while breastfeeding
Administration
Instruct patients and caregivers how to prepare and administer the correct dose of IMCIVREE and assess their ability to inject subcutaneously to ensure the proper administration of IMCIVREE. Instruct patients to use a 1 mL syringe with a 28- or 29-gauge needle appropriate for subcutaneous injection.
Manufactured for:
Rhythm Pharmaceuticals, Inc.
© 2024, Rhythm Pharmaceuticals, Inc. All rights reserved.
9PRINCIPAL DISPLAY PANEL - NDC: 72829-010-01 - Carton Label
10PRINCIPAL DISPLAY PANEL - NDC: 72829-010-01 - Vial Label