Brand Name
Clenpiq
Generic Name
Picosulfate
View Brand Information FDA approval date: March 29, 2023
Classification: Calculi Dissolution Agent
Form: Liquid
What is Clenpiq (Picosulfate)?
CLENPIQ is indicated for cleansing of the colon as a preparation for colonoscopy in adults and pediatric patients 9 years of age and older. CLENPIQ ® is a combination of sodium picosulfate, a stimulant laxative, and magnesium oxide and anhydrous citric acid, which form magnesium citrate, an osmotic laxative, indicated for cleansing of the colon as a preparation for colonoscopy in adults and pediatric patients ages 9 years and older.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Randomized, Assessor-Blind, Parallel-Groups, Multicenter Trial Assessing the Safety and Efficacy, Including Pharmacokinetic Assessments, of CLENPIQ in Children Aged 2 Years to Less Than 9 Years
Summary: Bowel preparation for pediatric colonoscopy.
Related Latest Advances
Brand Information
CLENPIQ (SODIUM PICOSULFATE, MAGNESIUM OXIDE, and ANHYDROUS CITRIC ACID)
1INDICATIONS AND USAGE
CLENPIQ is indicated for cleansing of the colon as a preparation for colonoscopy in adults and pediatric patients 9 years of age and older.
2DOSAGE FORMS AND STRENGTHS
Oral solution: Each bottle contains 10 mg of sodium picosulfate, 3.5 grams of magnesium oxide, and 12 grams of anhydrous citric acid in 175 mL of colorless to slightly yellow, clear solution with possible presence of visible particles.
3CONTRAINDICATIONS
CLENPIQ is contraindicated in the following conditions:
- Patients with severe renal impairment (creatinine clearance less than 30 mL/minute), which may result in accumulation of magnesium
- Gastrointestinal obstruction or ileus
- Bowel perforation
- Toxic colitis or toxic megacolon.
- Gastric retention.
- Hypersensitivity to any of the ingredients in CLENPIQ
4ADVERSE REACTIONS
The following serious or otherwise important adverse reactions for bowel preparations are described elsewhere in the labeling:
- Serious Fluid and Electrolyte Abnormalities
- Seizures
- Use in Patients with Renal Impairment
- Cardiac Arrhythmias
- Syncope
- Colonic Mucosal Ulceration, Ischemic Colitis and Ulcerative Colitis
- Use in Patients with Significant Gastrointestinal Disease
- Aspiration
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
4.2Postmarketing Experience
The following adverse reactions have been identified during post approval use of oral sodium picosulfate, magnesium oxide, and anhydrous citric acid products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity: rash, urticaria, and purpura
Gastrointestinal: abdominal pain, diarrhea, fecal incontinence, proctalgia, vomiting, reversible aphthoid ileal ulcers, and ischemic colitis [see
Neurologic: generalized tonic-clonic seizures with and without hyponatremia in epileptic patients, and syncope [see .
5OVERDOSAGE
Overdosage of more than the recommended dose of CLENPIQ may lead to severe electrolyte disturbances, as well as dehydration and hypovolemia, with signs and symptoms of these disturbances
6DESCRIPTION
CLENPIQ (sodium picosulfate, magnesium oxide, and anhydrous citric acid) oral solution is a stimulant and osmotic laxative that is provided as a cranberry-flavored, colorless to slightly yellow, clear solution with possible presence of visible particles. CLENPIQ is supplied as two bottles in each carton.
Each bottle of CLENPIQ contains 10 mg sodium picosulfate, USP; 3.5 g magnesium oxide, USP; and 12 g anhydrous citric acid, USP. The product also contains the following inactive ingredients:
acesulfame potassium, cranberry flavor, disodium edetate, malic acid, sodium benzoate, sodium hydroxide, sodium metabisulfite, sucralose, and water. The cranberry flavor contains glyceryl triacetate (triacetin), maltodextrin, and sodium octenyl succinated starch.
The following is a description of the three active ingredients contained in CLENPIQ:
Sodium picosulfate is a stimulant laxative.
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Instruct patients:
- CLENPIQ is ready to drink. It is a clear solution with possible presence of visible particles and it does not need to be diluted prior to administration. One bottle of CLENPIQ is equivalent to one dose.
- Two doses of CLENPIQ are required for a complete preparation for colonoscopy as a Split-Dose regimen. See
- Follow the directions in the
- Consume five or more 8-ounce cups of clear liquids after the first dose and four or more 8-ounce cups of clear liquids after the second dose
- Consume a variety of clear liquids after each dose of CLENPIQ. Ensure inclusion of balanced electrolyte solution, along with other clear liquids
- Consume only clear liquids (no solid food) on the day before colonoscopy and until after the colonoscopy.
- Do not eat solid food or dairy and do not drink anything colored red or purple.
- Do not drink alcohol.
- Do not take other laxatives while taking CLENPIQ.
- Administer oral medications at least one hour before starting each dose of CLENPIQ.
- If taking tetracycline or fluoroquinolone antibiotics, iron, digoxin, chlorpromazine, or penicillamine, take these medications at least 2 hours before and not less than 6 hours after administration of CLENPIQ.
- Delay the second dose of CLENPIQ, if severe bloating, distention, or abdominal pain occurs following the first dose until the symptoms resolve.
- Tell their healthcare provider if they have a history of fainting
- Contact their healthcare provider if they develop significant vomiting or signs of dehydration during or after taking CLENPIQ or if they experience confusion, delirium, dizziness, lightheadedness, fainting, loss of consciousness, or seizures
8Instructions for Use CLENPIQ®(CLEN-pik) (sodium picosulfate, magnesium oxide, and anhydrous citric acid) oral solution
READ BEFORE Taking CLENPIQ
If you have questions before you start CLENPIQ, talk to your healthcare provider.
Take CLENPIQ using the
- Drink the first bottle the evening before your colonoscopy.
- Drink the second bottle the morning of your colonoscopy.
Start a clear-liquid diet
- Drink only clear liquids all day the day before your colonoscopy.
- Drink only clear liquids the next day until 2 hours before your colonoscopy.
- Stop drinking all liquids at least 2 hours before your colonoscopy.
You
- Drink
- Drink
IMPORTANT: You can drink the clear liquids in Table 1.
IMPORTANT: Do NOT eat or drink the items in Table 2 starting from the day before your colonoscopy.
SPLIT-DOSE INSTRUCTIONS
DOSE 1
In the evening the day before your colonoscopy (sometime between 5:00 PM to 9:00 PM)
- Place the first bottle on a flat surface. Push down on the cap while twisting to the left (counterclockwise).
- Drink the entire first bottle of CLENPIQ. Drink CLENPIQ right from the bottle.
- Use the cup provided to drink five or more 8-ounce (oz) cups of clear liquids over the next 5 hours.
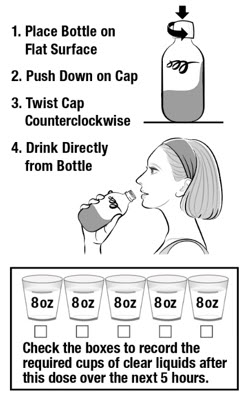
If you have any bloating or upset stomach after drinking CLENPIQ, wait until your stomach feels better before taking your second dose.
DOSE 2
In the morning of your colonoscopy (about 5 hours before your colonoscopy)
- Place the second bottle on a flat surface. Push down on the cap while twisting to the left (counterclockwise).
- Drink the entire second bottle of CLENPIQ. Drink CLENPIQ right from the bottle.
- Use the cup provided to drink four or more 8 ounce (oz) cups of clear liquids. You can continue to drink clear liquids up to 2 hours before the colonoscopy.
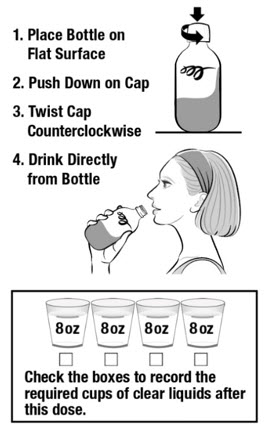
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Ferring Pharmaceuticals Inc.
9PRINCIPAL DISPLAY PANEL - 175 mL Bottle Carton
Cranberry
CLENPIQ
(sodium picosulfate, magnesium oxide,
10 mg/3.5 g/12 g per 175 mL bottle
CLENPIQ
Read the enclosed Instructions for Use and Medication Guide
Contains two (2) 175 mL bottles
Do not refrigerate or freeze.
Rx only
FERRING



