Brand Name
Xolremdi
Generic Name
Mavorixafor
View Brand Information FDA approval date: May 21, 2024
Form: Capsule
What is Xolremdi (Mavorixafor)?
XOLREMDI is indicated in patients 12 years of age and older with WHIM syndrome to increase the number of circulating mature neutrophils and lympocytes. XOLREMDI is a CXC chemokine receptor 4 antagonist indicated in patients 12 years of age and older with WHIM syndrome to increase the number of circulating mature neutrophils and lymphocytes.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Phase 3, Randomized, Double-blind, Placebo-controlled, Multicenter Study of Mavorixafor in Participants With Congenital and Acquired Primary Autoimmune and Idiopathic Chronic Neutropenic Disorders Who Are Experiencing Recurrent and/or Serious Infections
Summary: The purpose of this study is to demonstrate the efficacy and evaluate the safety and tolerability of mavorixafor in participants with congenital or acquired primary autoimmune and idiopathic chronic neutropenic disorders who are experiencing recurrent and/or serious infections as assessed by demonstrating its clinical benefit and increasing levels of circulating neutrophils.
An Open-label, Non-randomized Study to Evaluate the Pharmacokinetics (PK), Safety and Tolerability of a Single Dose of Mavorixafor in Participants With Hepatic Impairment (HI) Compared to Matched Healthy Volunteers With Normal Hepatic Function
Summary: The purpose of this study is to measure the effect of HI on the PK, safety, and tolerability of a single dose of mavorixafor compared to matched healthy volunteers (HVs) with normal hepatic function.
Related Latest Advances
Brand Information
XOLREMDI (mavorixafor)
1INDICATIONS AND USAGE
XOLREMDI is indicated in patients 12 years of age and older with WHIM syndrome (warts, hypogammaglobulinemia, infections and myelokathexis) to increase the number of circulating mature neutrophils and lympocytes.
2CONTRAINDICATIONS
Use of XOLREMDI is contraindicated with drugs that are highly dependent on CYP2D6 for clearance
3ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- QTc Interval Prolongation
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of XOLREMDI was evaluated in Study 1, a randomized placebo-controlled trial of 31 adult and pediatric patients 12 years and older with WHIM syndrome
The data below are based on the 52-week, placebo-controlled portion of the study. Twelve patients received XOLREMDI for at least 6 months, and 10 patients received XOLREMDI for at least 1 year.
Table 1 summarizes the most common adverse reactions (>10%) in Study 1, which were thrombocytopenia, pityriasis, rash, rhinitis, epistaxis, vomiting, and dizziness.
Serious adverse reactions of thrombocytopenia occurred in 3 of the 14 patients who received XOLREMDI, 2 of which occurred in the setting of infection or febrile neutropenia.
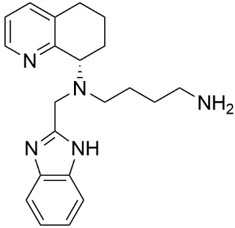
4DESCRIPTION
Mavorixafor is an orally bioavailable CXC Chemokine Receptor 4 (CXCR4) antagonist
The chemical name of the active ingredient, mavorixafor, is
Figure 1: Structural Formula
Mavorixafor is optically active and is a white to pale yellow to light brown solid. Mavorixafor is hygroscopic above relative humidities of 70%.
Mavorixafor is freely soluble in methanol, 95% ethanol and n-octanol, soluble in toluene, sparingly soluble in DMSO and acetonitrile, and very slightly soluble in HPLC grade water according to the USP solubility criteria.
Mavorixafor is soluble in pH 1.2 to 5.5 aqueous buffers and in pH 6.0 aqueous buffer, sparingly soluble in pH 6.8 aqueous buffer and slightly soluble in pH 7.5 aqueous buffer, according to the USP solubility criteria.
XOLREMDI is a hard gelatin capsule for oral administration. Each capsule contains 100 mg of mavorixafor with the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, dibasic calcium phosphate dihydrate, microcrystalline cellulose, sodium lauryl sulfate, and sodium stearyl fumarate. The hard gelatin capsule contains FD&C Blue #2, gelatin, and titanium dioxide. The Black Ink contains ammonium hydroxide 28%, ferrosoferric oxide/black iron oxide (E172), isopropyl alcohol, n-butyl alcohol, propylene glycol, and shellac glaze in ethanol.
5CLINICAL STUDIES
The efficacy of XOLREMDI in patients aged 12 and older with WHIM syndrome was demonstrated in the 52-week, randomized, double-blind, placebo-controlled portion of Study 1 [
Thirty-one patients were randomized 1:1 to receive either placebo (N=17) or XOLREMDI (N=14) once daily for 52 weeks. The efficacy of XOLREMDI in the treatment of patients with WHIM syndrome was based on improvement in absolute neutrophil counts (ANC), improvement in absolute lymphocyte counts (ALC), and a reduction in infections.
For ANC, the mean time (hours) above ANC threshold (TAT
1 - The results are based on an MMRM analysis with time above threshold as a dependent variable; treatment, visit (Weeks 13, 26, 39 and 52), treatment*visit, Ig use (randomization strata), and baseline time above threshold as covariates; and patient as the repeated random effect.
For ALC, the mean time (hours) above ALC threshold (TAT
The efficacy of XOLREMDI was further assessed in a composite endpoint consisting of total infection score and total wart change score using a Win-Ratio method (Table 4). The Win-Ratio of 2.76 is the number of pairs of XOLREMDI-treated patient "wins" divided by the number of pairs of placebo patient "wins."
Analyses of the individual components of this composite endpoint showed an approximately 40% reduction of total infection score, weighted by infection severity, in XOLREMDI-treated patients compared with placebo-treated patients. The annualized infection rate was reduced approximately 60% in XOLREMDI-treated patients [LS mean (SE) 1.7(0.5)] compared with placebo-treated patients [LS mean (SE) 4.2(0.7)]. There was no difference in total wart change scores between the XOLREMDI and placebo treatment arms over the 52-week period.
6HOW SUPPLIED/STORAGE AND HANDLING
XOLREMDI is supplied as an opaque white, hard gelatin capsule with a light blue cap, containing 100 mg of the active ingredient mavorixafor. The white capsule body is axially imprinted with "100 mg" in black ink, and the light blue capsule cap is axially imprinted with "MX4" in black ink.
XOLREMDI is supplied in child-resistant bottles as follows:
- 60 count– NDC 83296-100-60
- 90 count - NDC 83296-100-90
- 120 count– NDC 83296-100-12
7PRINCIPAL DISPLAY PANEL - 100 mg Capsule Bottle Label
NDC 83296-100-60
XOLREMDI™
100 mg
Swallow the capsules whole.
Rx only
60 capsules



