Brand Name
Xermelo
Generic Name
Telotristat
View Brand Information FDA approval date: March 01, 2017
Classification: Tryptophan Hydroxylase Inhibitor
Form: Tablet
What is Xermelo (Telotristat)?
Xermelo is indicated for the treatment of carcinoid syndrome diarrhea in combination with somatostatin analog therapy in adults inadequately controlled by SSA therapy. Xermelo is a tryptophan hydroxylase inhibitor indicated for the treatment of carcinoid syndrome diarrhea in combination with somatostatin analog therapy in adults inadequately controlled by SSA therapy.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Xermelo (telotristat ethyl)
1INDICATIONS AND USAGE
Xermelo is indicated for the treatment of carcinoid syndrome diarrhea in combination with somatostatin analog (SSA) therapy in adults inadequately controlled by SSA therapy.
2DOSAGE AND ADMINISTRATION
The recommended dosage of Xermelo in adult patients is 250 mg three times daily for patients whose diarrhea is inadequately controlled by SSA therapy.
Administration
- Take Xermelo with food
- When short-acting octreotide is used in combination with Xermelo, administer short-acting octreotide at least 30 minutes after administering Xermelo
- If a dose is missed, take the next dose at the regular time. Do not take 2 doses at the same time to make up for a missed dose.
- Discontinue Xermelo if severe constipation develops
3DOSAGE FORMS AND STRENGTHS
Tablets: 250 mg telotristat ethyl; white to off-white, coated and oval with “T-E” debossed on one side and “250” debossed on the other side.
4CONTRAINDICATIONS
Xermelo is contraindicated in patients with a history of a hypersensitivity reaction to telotristat. Reactions have included angioedema, rash and pruritis.
5DESCRIPTION
Xermelo (telotristat ethyl) tablets contain telotristat ethyl as telotristat etiprate, a tryptophan hydroxylase inhibitor. Telotristat etiprate is the hippurate salt of telotristat ethyl [(S)-ethyl 2-amino-3-(4-(2-amino-6-((R)-1-(4-chloro-2-(3-methyl-1H-pyrazol-1-yl)phenyl)-2,2,2-trifluoroethoxy)pyrimidin-4-yl)phenyl)propanoate], which undergoes hydrolysis to the active metabolite, (S)-2-amino-3-(4-(2-amino-6-((R)-1-(4-chloro-2-(3-methyl-1H-pyrazol-1-yl)phenyl)-2,2,2-trifluoroethoxy)pyrimidin-4-yl)phenyl)propanoic acid.
The molecular formula of telotristat etiprate is C
Chemical Structure:
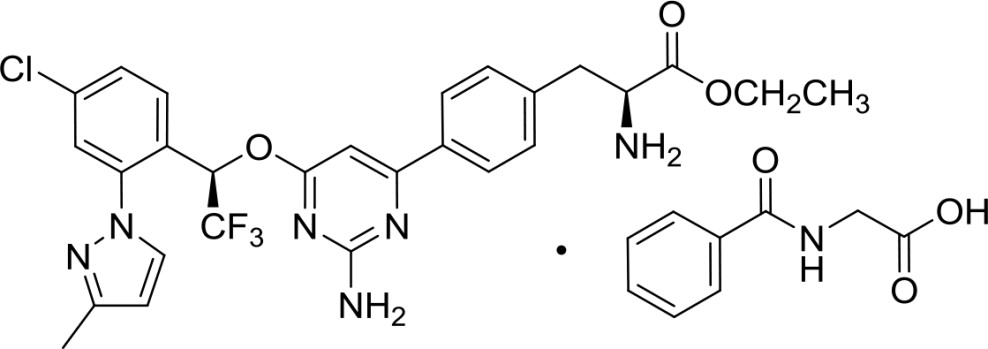
Telotristat etiprate is a white to off-white solid. The solubility is a function of pH at 25°C; at pH 1 (0.1N HCl), the solubility is greater than 71 mg/mL, at pH 3 phosphate buffer, the solubility is 0.30 mg/mL, at a pH of 5 to 9, the solubility is negligible. In organic solvents, telotristat etiprate is freely soluble in methanol, soluble in acetone, and sparingly soluble in ethanol.
Each Xermelo tablet contains 250 mg of telotristat ethyl (free base) which is equivalent to 328 mg telotristat etiprate. The inactive ingredients of Xermelo tablets include: colloidal silicon dioxide, croscarmellose sodium, hydroxypropyl cellulose, lactose anhydrous, macrogol/PEG, magnesium stearate, polyvinyl alcohol [part hydrolyzed], talc and titanium dioxide.
6CLINICAL STUDIES
A 12-week double-blind, placebo-controlled, randomized, multicenter trial of Xermelo was conducted in adult patients with a well-differentiated metastatic neuroendocrine tumor and carcinoid syndrome diarrhea who were having between 4 to 12 daily bowel movements despite the use of SSA therapy at a stable dose for at least 3 months. Patients were randomized to placebo or treatment with Xermelo 250 mg three times daily.
Study medication was administered within 15 minutes before or within 1 hour after a meal or snack
The primary efficacy endpoint was the change from baseline in the number of daily bowel movements averaged over the 12-week treatment period. The analysis results can be found in
In the 12-week study, a difference in average weekly reductions in bowel movement frequency between Xermelo and placebo was observed as early as 1 to 3 weeks, and persisted for the remaining 9 weeks of the study.
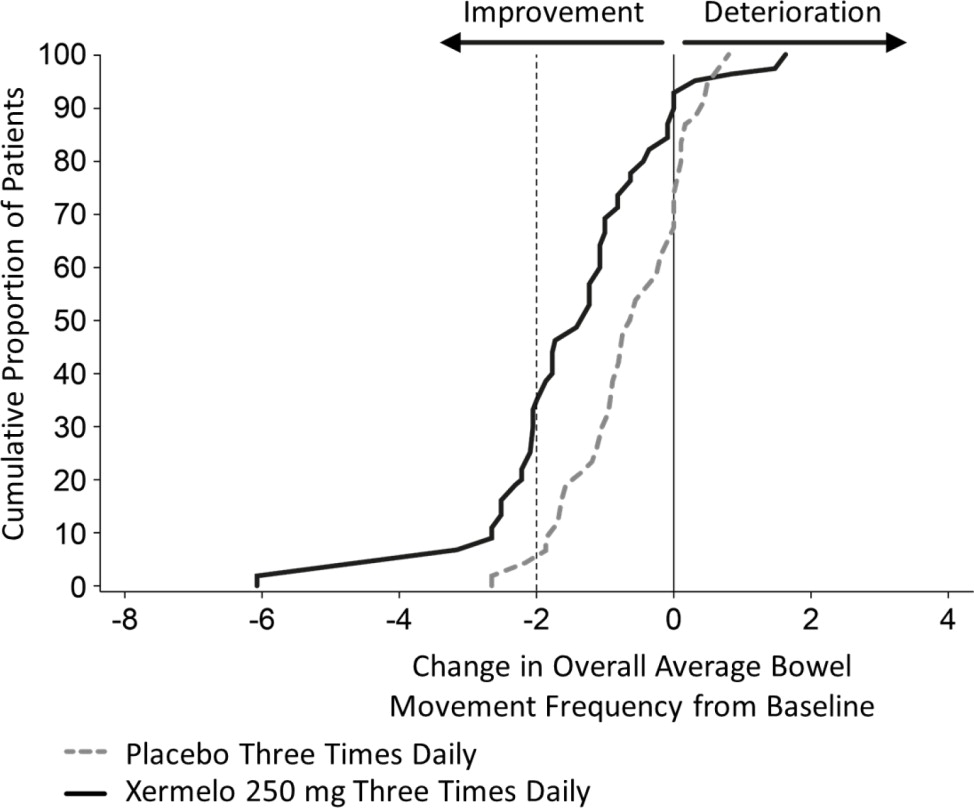
To aid in the interpretation of the bowel movement reduction results, the proportion of patients reporting any particular level of reduction in overall average bowel movement frequency is depicted in Figure 1 below. For example, 33% of patients randomized to Xermelo and 4% of patients randomized to placebo experienced a reduction in overall average bowel movements from baseline of at least 2 bowel movements per day.
Figure 1: Cumulative Proportion of Patients with Carcinoid Syndrome Diarrhea Reporting Change in Overall Average Bowel Movement Frequency
Other symptoms of carcinoid syndrome (abdominal pain or flushing) did not show improvement in the comparison of Xermelo to placebo.
The average number of daily short-acting octreotide injections used for rescue therapy over the 12-week double-blind treatment period was 0.3 and 0.7 in the Xermelo and placebo groups, respectively. In the subgroup of patients who received short-acting octreotide injections, observed reductions in the number of bowel movements per day and treatment differences were generally consistent with the reductions and differences observed in patients who did not receive rescue therapy, and were similar to the overall data presented in
A third randomized treatment arm of Xermelo 500 mg three times daily did not demonstrate additional treatment benefit on the primary endpoint and had a greater incidence of adverse reactions than Xermelo 250 mg three times daily. Therefore, Xermelo 500 mg three times daily is not recommended
7PATIENT COUNSELING INFORMATION
Advise patients:
- If they experience severe constipation or severe persistent or worsening abdominal pain, to discontinue Xermelo and contact their healthcare provider
- To take Xermelo with food
- When short-acting octreotide is used in combination with Xermelo, administer short-acting octreotide at least 30 minutes after administering Xermelo
- If a dose is missed, take the next dose at the regular time. Do not take 2 doses at the same time to make up for a missed dose.
Distributed by:
©2022 TerSera Therapeutics LLC



