Rexulti
What is Rexulti (Brexpiprazole)?
Approved To Treat
Related Clinical Trials
Summary: Study to evaluate the safety and tolerability of single ascending doses of brexpiprazole long-acting injection in healthy subjects/patients with schizophrenia.
Summary: MainRexult study aims to carefully evaluate a cohort of patients with schizophrenia and related disorder prescribed with the combination therapy with Abilify Maintena and Rexulti on its efficacy and tolerability in a real-life clinical setting.
Summary: Not only being the mainstay of treatment for schizophrenia spectrum psychotic disorders, antipsychotics, especially the second-generation antipsychotics (SGAs) have also been recommended as augmenting agents for treating depression. Dopaminergic agents, including both dopamine D2/D3 antagonists and dopamine partial D2 agonists, have been effective for treating psychosis and schizophrenia. Amongst ...
Related Latest Advances
Brand Information
- Adjunctive treatment to antidepressants for major depressive disorder (MDD) in adults
- Treatment of schizophrenia in adults and pediatric patients ages 13 years and older
- Treatment of agitation associated with dementia due to Alzheimer's disease
- 0.25 mg tablets are light brown, round, shallow convex, bevel-edged body with "BRX" and "0.25" imprinted on one side.
- 0.5 mg tablets: are light orange, round, shallow convex, bevel-edged body with "BRX" and "0.5" imprinted on one side.
- 1 mg tablets are light yellow, round, shallow convex, bevel-edged body with "BRX" and "1" imprinted on one side.
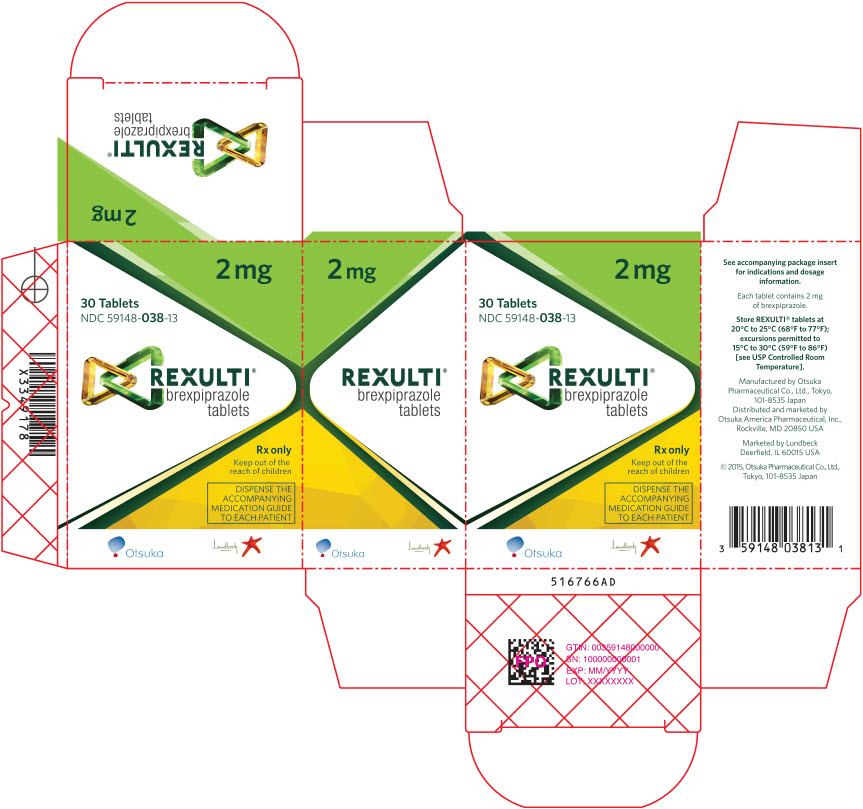
- 2 mg tablets are light green, round, shallow convex, bevel-edged body with "BRX" and "2" imprinted on one side.
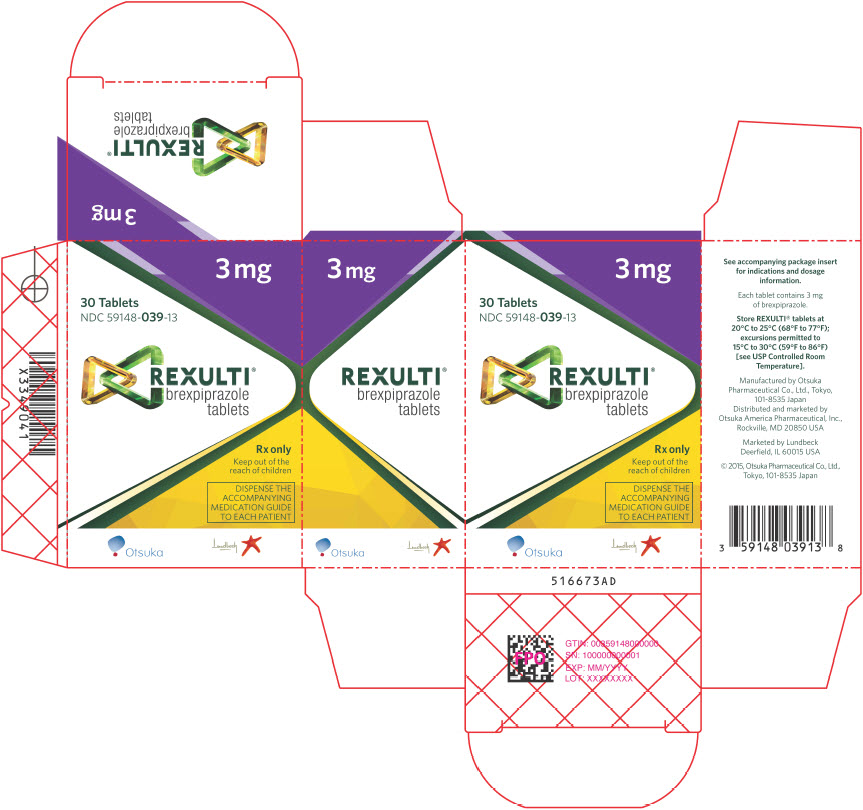
- 3 mg tablets are light purple, round, shallow convex, bevel-edged body with "BRX" and "3" imprinted on one side.
- 4 mg tablets are white, round, shallow convex, bevel-edged body with "BRX" and "4" imprinted on one side.
- Increased Mortality in Elderly Patients with Dementia-Related Psychosis
- Suicidal Thoughts and Behaviors in Adolescents and Young Adults
- Cerebrovascular Adverse Reactions Including Stroke in Elderly Patients with Dementia-Related Psychosis
- Neuroleptic Malignant Syndrome (NMS)
- Tardive Dyskinesia
- Metabolic Changes
- Pathological Gambling and Other Compulsive Behaviors
- Leukopenia, Neutropenia, and Agranulocytosis
- Orthostatic Hypotension and Syncope
- Falls
- Seizures
- Body Temperature Dysregulation
- Dysphagia
- Potential for Cognitive and Motor Impairment
NDC 59148-035-13
NDC 59148-036-13
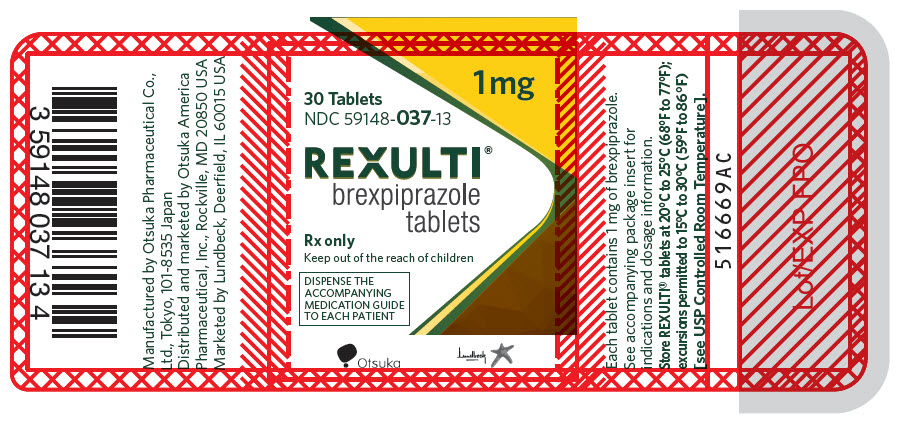
NDC 59148-037-13
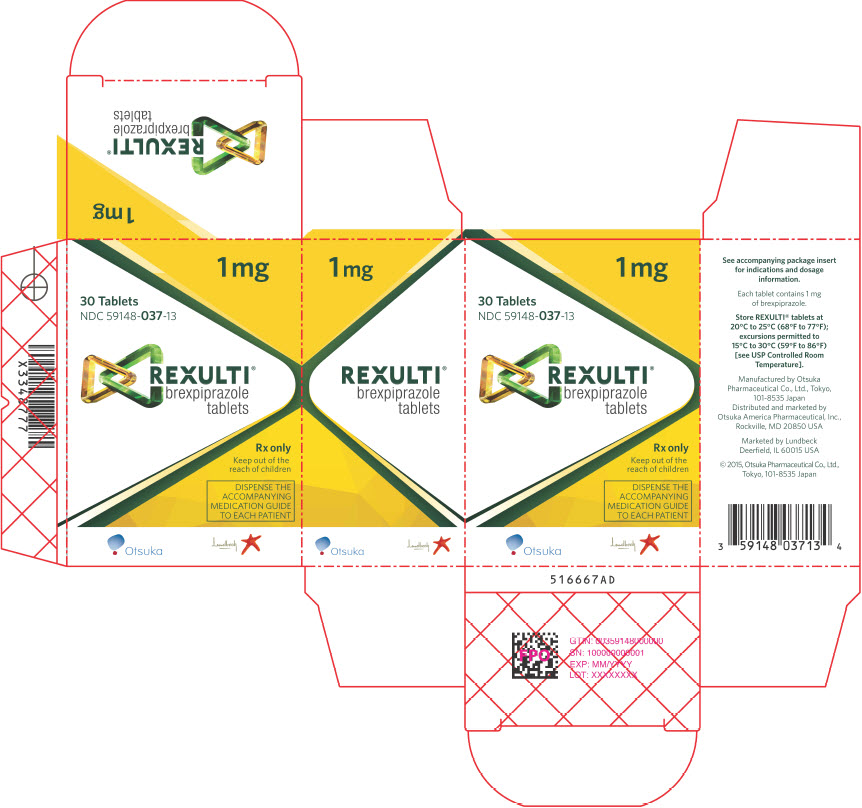
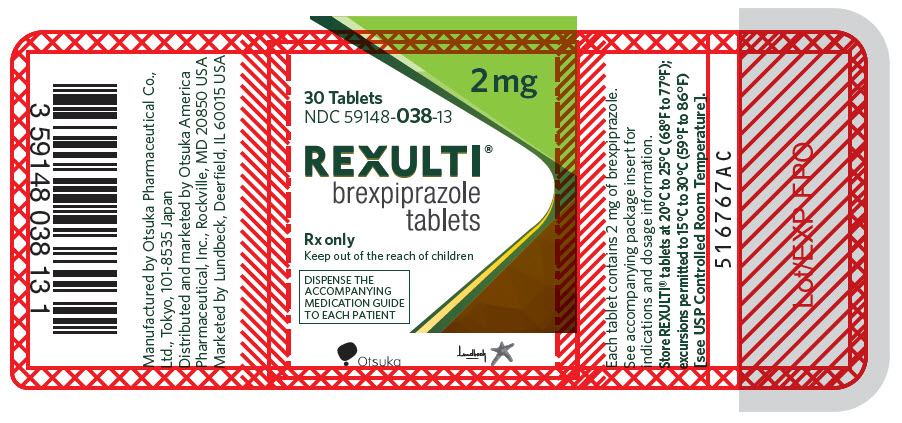
NDC 59148-038-13
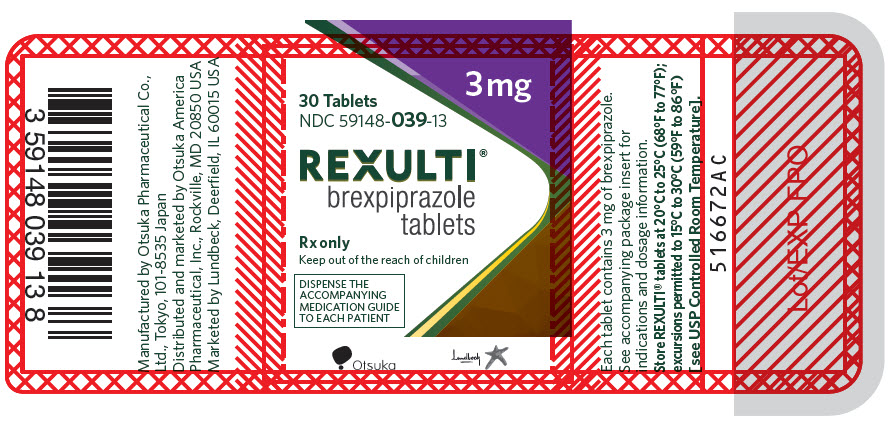
NDC 59148-039-13
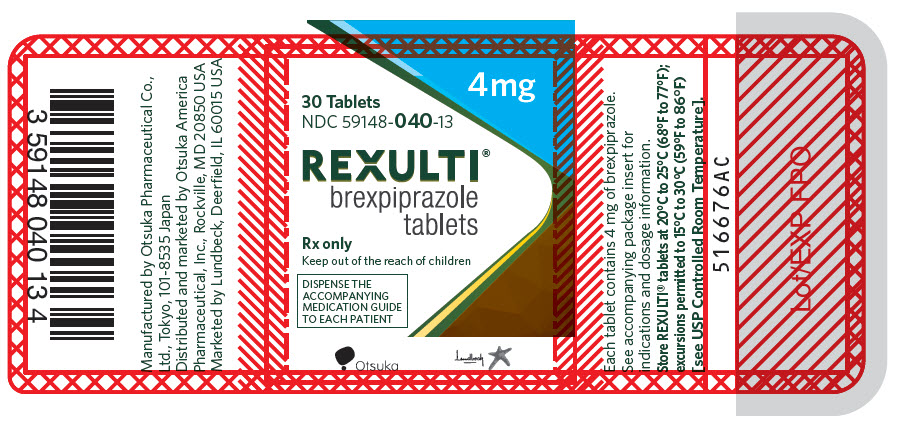
NDC 59148-040-13