Brand Name
Rezdiffra
Generic Name
Resmetirom
View Brand Information FDA approval date: March 14, 2024
Classification: Thyroid Hormone Receptor beta Agonist
Form: Tablet
What is Rezdiffra (Resmetirom)?
REZDIFFRA is indicated in conjunction with diet and exercise for the treatment of adults with noncirrhotic nonalcoholic steatohepatitis with moderate to advanced liver fibrosis . This indication is approved under accelerated approval based on improvement of NASH and fibrosis. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. Limitations of Use Avoid use of REZDIFFRA in patients with decompensated cirrhosis [see Use in Specific Populations.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Clinical Evaluation of Safety and Efficacy of Resmiterom in Patients With MASH (Metabolic Dysfunction-Associated Steato-Hepatitis) A Prospective, Open-label, Interventional Study
Summary: Phase 4 clinical trial study aims to further evaluate the safety and therapeutic efficacy of Resmetirom in Pakistani patients with fibroscan proven MASH.
Related Latest Advances
Brand Information
1INDICATIONS AND USAGE
REZDIFFRA is indicated in conjunction with diet and exercise for the treatment of adults with noncirrhotic nonalcoholic steatohepatitis (NASH) with moderate to advanced liver fibrosis (consistent with stages F2 to F3 fibrosis).
This indication is approved under accelerated approval based on improvement of NASH and fibrosis
Limitations of Use
Avoid use of REZDIFFRA in patients with decompensated cirrhosis
2DOSAGE FORMS AND STRENGTHS
REZDIFFRA Tablets:
- 60 mg: white oval-shaped film-coated tablets debossed with “P60” on one side and plain on the other side.
- 80 mg: yellow, oval-shaped, film-coated tablets debossed with “P80” on one side and plain on the other side.
- 100 mg: beige to pink, oval-shaped, film-coated tablets debossed with “P100” on one side and plain on the other side.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
- Hepatotoxicity
- Gallbladder-Related Adverse Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of REZDIFFRA was evaluated in two randomized, double-blind, placebo-controlled trials that enrolled a total of 2019 patients.
Trial 1
Trial 1 included patients who had noncirrhotic NASH with stages F2 and F3 fibrosis at eligibility (n=888)
Adverse Reactions Leading to Discontinuations
The exposure-adjusted incidence rates (EAIRs) per 100 person-years (PY) for treatment discontinuation due to any adverse reaction were higher in the REZDIFFRA dosage arms: 4 per 100 PY, 5 per 100 PY, and 8 per 100 PY in placebo, REZDIFFRA 80 mg once daily, and REZDIFFRA 100 mg once daily arms, respectively. Diarrhea and nausea were the most common causes of treatment discontinuation.
Common Adverse Reactions
Table 1 displays EAIRs per 100 PY for the common adverse reactions that occurred in at least 5% of patients with F2 or F3 fibrosis treated in either drug arm with REZDIFFRA and were greater than that reported for placebo.
Gastrointestinal Adverse Reactions
The incidence of gastrointestinal adverse reactions was higher for the REZDIFFRA drug arms compared to placebo. The EAIRs for gastrointestinal adverse reactions were 57 per 100 PY, 73 per 100 PY, and 89 per 100 PY in the placebo, REZDIFFRA 80 mg once daily, REZDIFFRA 100 mg once daily arms, respectively.
Diarrhea typically began early in treatment initiation and was mild to moderate in severity. The median time (Q1 to Q3) to a diarrheal event was 39 (2 to 195) days, 17 (3 to 70) days, and 6 (2 to 54) days in the placebo, REZDIFFRA 80 mg once daily, and REZDIFFRA 100 mg once daily arms, respectively.
Median duration of diarrhea was 9 days for placebo compared to 20 days for both REZDIFFRA 80 mg once daily and REZDIFFRA 100 mg once daily dosage arms.
Nausea also began early in treatment and was mild to moderate in severity. Among patients with nausea, the median time (Q1 to Q3) to a nausea event was 85 (24 to 347) days, 28 (2 to 162) days, and 5 (2 to 40) days in the placebo, REZDIFFRA 80 mg once daily, and REZDIFFRA 100 mg once daily arms, respectively. Median duration of nausea was 17 days, 26 days, and 28 days for patients in the placebo, REZDIFFRA 80 mg once daily, and REZDIFFRA 100 mg once daily arms, respectively.
Vomiting and abdominal pain adverse reactions were mild to moderate in severity.
Hypersensitivity Reactions
Reactions such as urticaria and rash, which may reflect drug hypersensitivity, were observed in patients receiving REZDIFFRA. The EAIRs for urticaria were 0.2 per 100 PY, 0.7 per 100 PY, and 1.5 per 100 PY in the placebo, REZDIFFRA 80 mg once daily, and REZDIFFRA 100 mg once daily arms, respectively. The EAIRs for rash were 3 per 100 PY in the placebo and REZDIFFRA 80 mg once daily arms compared to 5 per 100 PY in the REZDIFFRA 100 mg once daily arm.
Gallbladder-Related Adverse Reactions
A higher incidence of cholelithiasis, acute cholecystitis, and obstructive pancreatitis (gallstone) was observed in the treatment arms compared to placebo. However, the EAIRs for these events were less than 1 per 100 PY for all treatment arms.
Less Common Adverse Reactions
Additional adverse reactions that occurred more frequently in the REZDIFFRA arms compared to placebo, in less than 5% of patients, included decreased appetite, flatulence, abnormal feces, dysgeusia, vertigo, arrhythmia, palpitations, depression, erythema, hypoglycemia, tendinopathy, abnormal uterine bleeding.
Laboratory Abnormalities
Liver Tests
Increases in mean alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels
Table 2 presents the frequency of liver test elevations during Trial 1.
Thyroid Function Tests
A decrease in levels of prohormone free T4 (FT4) of mean 2%, 13%, and 17% was seen at 12 months in patients treated with placebo, REZDIFFRA 80 mg once daily, and REZDIFFRA 100 mg once daily, respectively, with minimal changes in active hormone T3 or in TSH. There were no clinical findings associated with FT4 decreases.
Additional Safety Data
The safety evaluation of REZDIFFRA also included an analysis of an additional randomized placebo-controlled safety trial which included 969 patients from a relevant patient population (placebo [n=318], REZDIFFRA 80 mg once daily [n=327], and REZDIFFRA 100 mg once daily [n=324]).
Data from the safety trial was combined with data from NASH patients with F2 and F3 fibrosis at eligibility (n=888) and data from an additional 162 patients from a relevant patient population enrolled in Trial 1. In the combined safety population (n=2019), the median (Q1 to Q3) age of patients at baseline was 58 (50 to 65) years; 55% were female, 28% were Hispanic, 89% were White, 2% were Asian, and 4% were Black or African American.
The safety profile from this combined analysis was similar to that in Trial 1, other than the one case of hepatotoxicity in the safety trial
5DESCRIPTION
REZDIFFRA (resmetirom) tablets contain resmetirom, a thyroid hormone receptor-beta agonist. The chemical name for REZDIFFRA is 2-[3,5-Dichloro-4-((6-oxo-5-(propan-2-yl)-1,6-dihydropyridazin-3-yl)oxy)phenyl]-3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile. The molecular formula is C
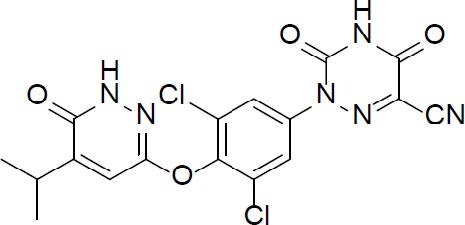
Resmetirom has low aqueous solubility below pH 6 and higher solubility above pH 7 (0.44 mg/mL at pH 7.04).
REZDIFFRA tablets are supplied in 60 mg, 80 mg, and 100 mg strengths for oral administration. Each tablet contains the active ingredient, resmetirom, and the following USP/NF excipients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, mannitol, and microcrystalline cellulose. REZDIFFRA tablets are film-coated with an Opadry coating comprised of polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, red iron oxide (100 mg tablets), yellow iron oxide (80 mg and 100 mg tablets).
6CLINICAL STUDIES
The efficacy of REZDIFFRA was evaluated based on an efficacy analysis at Month 12 in Trial 1 (
The month 12 analysis included 888 F2 and F3 (at eligibility) patients randomized 1:1:1 to receive placebo (n = 294), REZDIFFRA 80 mg once daily (n = 298), or REZDIFFRA 100 mg once daily (n = 296), in addition to lifestyle counseling on nutrition and exercise. Patients were on stable doses of medications for diabetes, dyslipidemia, and hypertension.
Demographic and baseline characteristics were balanced between treatment and placebo groups. Overall, the median (Q1 to Q3) age of patients at baseline was 58 (51 to 65) years, 56% were female, 21% were Hispanic, 89% were White, 3% were Asian, and 2% were Black or African American.
Table 8 presents the Month 12 histopathology results comparing REZDIFFRA with placebo on 1) the percentage of patients with resolution of steatohepatitis and no worsening of liver fibrosis and 2) the percentage of patients with at least one stage improvement in liver fibrosis and no worsening of steatohepatitis. Two pathologists, Pathologist A and Pathologist B, independently read the liver biopsies for each patient. Both the 80 mg once daily and the 100 mg once daily dosages of REZDIFFRA demonstrated improvement on these histopathology endpoints at Month 12 compared to placebo. In a statistical analysis incorporating both pathologists’ independent readings, REZDIFFRA achieved statistical significance on both histopathology endpoints for both doses.
Examination of age, gender, diabetes status (Yes or No), and fibrosis stage (F2 or F3) subgroups did not identify differences in response to REZDIFFRA among these subgroups. The majority of patients in the trial were white (89%); there were too few patients of other races to adequately assess differences in response by race.
Starting at Month 3 and through Month 12, there was a trend of greater reductions from baseline in average ALT and AST in the REZDIFFRA groups as compared to the placebo group.
7HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
REZDIFFRA (resmetirom) tablets are packaged in white high-density polyethylene bottles closed with a child-resistant closure containing an induction seal.
60 mg Tablets: white oval-shaped film-coated tablets, debossed “P60” on one side and plain on the other side.
- Bottle of 30 count (NDC 82576-060-30)
80 mg Tablets: yellow, oval-shaped, film-coated tablets, debossed with “P80” on one side and plain on the other side.
- Bottle of 30 count (NDC 82576-080-30)
- Bottle of 90 count (NDC 82576-080-90)
100 mg Tablets: beige to pink, oval-shaped, film-coated tablets, debossed with “P100” on one side and plain on the other side.
- Bottle of 30 count (NDC 82576-100-30)
- Bottle of 90 count (NDC 82576-100-90)
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Administration Instructions
Instruct patients to swallow REZDIFFRA tablets whole and not split, crush, or chew tablets [see ].
Instruct patients to swallow REZDIFFRA tablets whole and not split, crush, or chew tablets [see ].
Hepatotoxicity
Inform patients of the risk of hepatotoxicity. Instruct patients to immediately report any signs or symptoms of severe liver injury (e.g., fatigue, nausea, vomiting, right upper quadrant pain or tenderness, jaundice, fever, rash) to their healthcare provider
Gallbladder-Related Adverse Reactions
Inform patients of the potential risk for cholelithiasis, cholecystitis, and obstructive pancreatitis (gallstones) during treatment with REZDIFFRA. Instruct patients to contact their healthcare provider if they develop signs or symptoms of these conditions
Drug Interaction with Statins
Inform patients that concomitant use of REZDIFFRA with some statins may increase the risk of statin- related adverse reactions (e.g., elevation of liver tests, myopathy, rhabdomyolysis)
Pregnancy
Inform patients that there is a pregnancy safety study that monitors pregnancy outcomes in women exposed to REZDIFFRA during pregnancy. Encourage patients to report their pregnancy by visiting https://pregnancyregistry.madrigalpharma.com or calling 1-800-905-0324 [
Manufactured for and Distributed by:
Madrigal Pharmaceuticals, Inc.
West Conshohocken, PA
REZDIFFRA™ (resmetirom)
© 2025 Madrigal Pharmaceuticals, Inc.


