Brand Name
Agamree
Generic Name
Vamorolone
View Brand Information FDA approval date: November 23, 2023
Form: Kit, Suspension
What is Agamree (Vamorolone)?
AGAMREE is a corticosteroid indicated for the treatment of Duchenne muscular dystrophy in patients 2 years of age and older. AGAMREE is indicated for the treatment of Duchenne muscular dystrophy in patients 2 years of age and older.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Registry Study to Observe Long-term Safety of Vamorolone (AGAMREE®) in Patients With Duchenne Muscular Dystrophy
Summary: The goal of this observational study is to follow patients being treated with the FDA approved drug AGAMREE® in male patients 2 years of age or older with Duchenne's Muscular Dystrophy for long term safety and quality of life.
Related Latest Advances
Brand Information
AGAMREE (VAMOROLONE)
1INDICATIONS AND USAGE
AGAMREE is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients 2 years of age and older.
2DOSAGE FORMS AND STRENGTHS
Oral Suspension: 40 mg/mL orange flavored white to off-white suspension
3CONTRAINDICATIONS
AGAMREE is contraindicated in patients with known hypersensitivity to vamorolone or to any of the inactive ingredients of AGAMREE. Instances of hypersensitivity, including anaphylaxis, have occurred in patients receiving corticosteroid therapy
4ADVERSE REACTIONS
The following serious adverse reactions are discussed in more detail in other sections:
- Alterations in Endocrine Function
- Immunosuppression and Increased Risk of Infection
- Alterations in Cardiovascular/Renal Function
- Gastrointestinal Perforation
- Behavioral and Mood Disturbances
- Effects on Bones
- Ophthalmic Effects
- Immunizations
- Effects on Growth and Development
- Myopathy
- Kaposi's Sarcoma
- Thromboembolic Events
- Anaphylaxis
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
5OVERDOSAGE
Treatment of acute overdosage of vamorolone is by immediate supportive and symptomatic therapy. Gastric lavage or emesis can be considered.
6DESCRIPTION
AGAMREE (vamorolone) oral suspension contains vamorolone, a corticosteroid. Vamorolone [17α,21-dihydroxy-16α-methyl-pregna-1,4,9(11)-triene-3,20-dione] is a white to off-white powder with a molecular formula of C
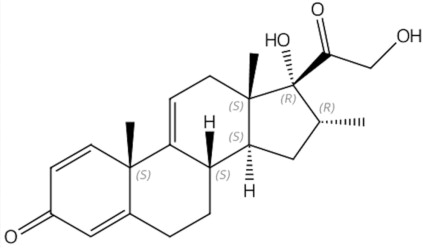
Vamorolone is freely soluble in methanol and dioxane and sparingly soluble in ethanol and acetone.
AGAMREE for oral administration is available as an oral suspension in a strength of 40 mg/mL. The oral suspension contains vamorolone and the following inactive ingredients: citric acid (monohydrate), disodium phosphate, glycerin, hydrochloric acid (for pH adjustment), orange flavor, sodium benzoate, sucralose, water, and xanthan gum.
7CLINICAL STUDIES
The effectiveness of AGAMREE for the treatment of Duchenne muscular dystrophy (DMD) was evaluated in a multicenter, randomized, double-blind, parallel-group, placebo- and active-controlled, multinational 24-week study (Study 1; NCT03439670). The study randomized 121 male patients with DMD to one of the following treatment groups: AGAMREE 6 mg/kg/day (n=30), AGAMREE 2 mg/kg/day (n=30), prednisone 0.75 mg/kg/day (n=31), or placebo (n=30) for 24 weeks. After 24 weeks, patients on prednisone and placebo received either AGAMREE 6 mg/kg/day (n=29) or AGAMREE 2 mg/kg/day (n=29) for an additional 20 weeks. The study included patients 4 to less than 7 years of age at time of enrollment in the study who were corticosteroid naïve and ambulatory, with a confirmed diagnosis of DMD. At baseline, patients had a mean age of 5.4 years, 83% were Caucasian, 10% were Asian, and 96% were not Hispanic or Latino.
The primary endpoint was the change from baseline to Week 24 in Time to Stand Test (TTSTAND) velocity for AGAMREE 6 mg/kg/day compared to placebo. TTSTAND velocity is a measure of muscle function that measures the time required for the patient to stand to an erect position from a supine position (floor). The key secondary endpoints consisted of change from baseline to Week 24 in TTSTAND velocity (AGAMREE 2 mg/kg/day vs placebo), 6 Minute Walk Test (6MWT) distance (AGAMREE 6 mg/kg/day vs placebo and 2 mg/kg/day vs placebo) and Time to Run/Walk 10 meters (TTRW) velocity (AGAMREE 6 mg/kg/day vs placebo and 2 mg/kg/day vs placebo). The 6MWT measures the distance that a patient can walk on a flat, hard surface in a period of 6 minutes and TTRW measures the time that it takes a patient to run or walk 10 meters. The fixed sequential testing process was applied to the key secondary endpoints in the order listed above.
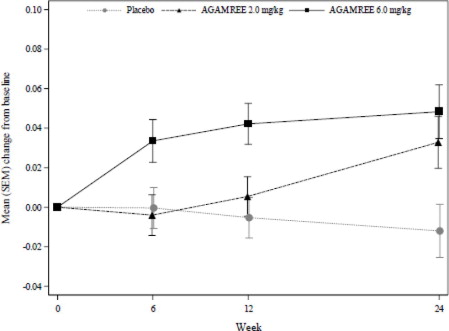
The primary endpoint and key secondary endpoints were met for the AGAMREE 6 mg/kg/day treatment group. The AGAMREE 2 mg/kg/day treatment group was statistically significant vs. placebo for TTSTAND and 6MWT, but was not statistically significant vs. placebo for TTRW. See
Figure 1: Least-Squares Mean Change in Time to Stand (TTSTAND) Velocity (rises/sec)
8PATIENT COUNSELING INFORMATION
Advise the patients and/or caregivers to read the FDA-approved patient labeling (
