Brand Name
Wainua
Generic Name
Eplontersen
View Brand Information FDA approval date: December 21, 2023
Classification: Transthyretin-directed RNA Interaction
Form: Injection
What is Wainua (Eplontersen)?
WAINUA is indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults. WAINUA is a transthyretin-directed antisense oligonucleotide indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Non-interventional, Prospective, Multi-country Study Collecting Real-world Data on the Characteristics, Treatment Patterns, and Outcomes of Patients With Transthyretin (ATTR) Amyloidosis
Summary: The MaesTTRo study aims to enroll a global cohort of patients with transthyretin (ATTR) amyloidosis to longitudinally observe the natural course of the disease and describe real-world treatment patterns and outcomes. In addition, information on the effectiveness of ATTR amyloidosis treatments, including eplontersen, which is a ligand-conjugated antisense oligonucleotide gene silencing treatment ta...
Related Latest Advances
Brand Information
WAINUA (EPLONTERSEN)
1INDICATIONS AND USAGE
WAINUA is indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults.
2DOSAGE FORMS AND STRENGTHS
Injection: 45 mg/0.8 mL of eplontersen as a clear, colorless-to-yellow solution in a single-dose autoinjector.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Reduced Serum Vitamin A Levels and Recommended Supplementation
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of WAINUA cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In Study 1
Table 1 lists the adverse reactions that occurred in at least 5% of patients treated with WAINUA in Study 1.
Three serious adverse reactions of atrioventricular (AV) heart block (2%) occurred in WAINUA-treated patients, including 1 case of complete AV block.
Laboratory Tests
Vitamin A Decrease
In Study 1, patients were instructed to take the recommended daily allowance of vitamin A
5DESCRIPTION
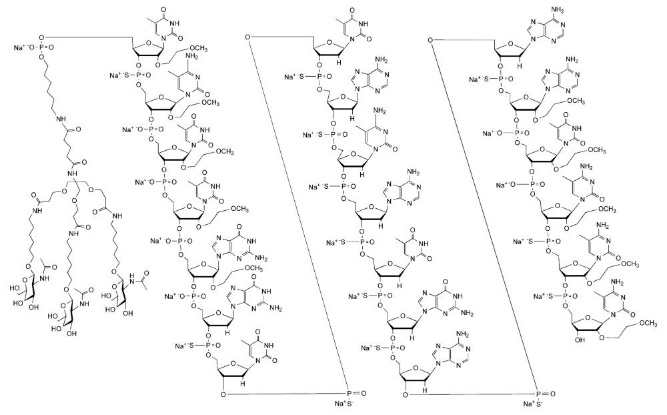
Eplontersen is a transthyretin-directed antisense oligonucleotide (ASO), covalently linked to a ligand containing three N-acetyl galactosamine (GalNAc) residues to enable delivery of the ASO to hepatocytes.
WAINUA contains eplontersen sodium as the active ingredient. Eplontersen sodium is a white to yellow solid and it is freely soluble in water and in phosphate buffer. The molecular formula of eplontersen sodium is C
The structure of eplontersen sodium is presented below:
WAINUA is a sterile, preservative-free, aqueous solution for subcutaneous injection. Each single-dose autoinjector contains 45 mg eplontersen (equivalent to 47 mg eplontersen sodium) in 0.8 mL of solution. The solution also contains 0.868 mg dibasic sodium phosphate, anhydrous (buffering agent); 0.238 mg monobasic sodium phosphate, dihydrate (buffering agent); 4.2 mg sodium chloride (tonicity modifier); water for injection; and may include hydrochloric acid and/or sodium hydroxide for pH adjustment between 6.9 - 7.9. Each dose of WAINUA injection contains less than 5 mg of sodium and less than 5 mg of phosphorus.
6CLINICAL STUDIES
The efficacy of WAINUA was demonstrated in a randomized, open-label, multicenter clinical trial in adult patients with polyneuropathy caused by hATTR amyloidosis (Study 1; NCT04136184). Patients were randomized in a 6:1 ratio to receive either 45 mg of WAINUA once every 4 weeks (N=144), or 284 mg of inotersen once per week (N=24), respectively, as subcutaneous injections. Ninety-seven percent of WAINUA-treated patients and 83% of inotersen-treated patients completed at least 35 weeks of the assigned treatment.
Efficacy assessments were based on a comparison of the WAINUA arm of Study 1 with an external placebo group (N=60) in another study (NCT01737398) composed of a comparable population of adult patients with polyneuropathy caused by hATTR amyloidosis.
The efficacy endpoints were the change from baseline to Week 35 in the modified Neuropathy Impairment Scale+7 (mNIS+7) composite score and the change from baseline to Week 35 in the Norfolk Quality of Life-Diabetic Neuropathy (QoL-DN) total score.
The mNIS+7 is an objective assessment of neuropathy and comprises the neuropathy impairment score (NIS) and Modified +7 composite scores. In the version of the mNIS+7 used in the trial, the NIS objectively measures deficits in cranial nerve function, muscle strength, reflexes, and sensations, and the Modified +7 assesses heart rate response to deep breathing, quantitative sensory testing (touch-pressure and heat-pain), and peripheral nerve electrophysiology. The validated version of the mNIS+7 score used in the trial has a range of -22.3 to 346.3 points, with higher scores representing a greater severity of disease.
The clinical meaningfulness of effects on the mNIS+7 was assessed by the change from baseline to Week 35 in Norfolk Quality of Life-Diabetic Neuropathy (QoL-DN) total score. The Norfolk QoL-DN scale is a patient-reported assessment that evaluates the subjective experience of neuropathy in the following domains: physical functioning/large fiber neuropathy, activities of daily living, symptoms, small fiber neuropathy, and autonomic neuropathy. The version of the Norfolk QoL-DN that was used in the trial has a range from -4 to 136 points, with higher scores representing greater impairment.
Treatment with WAINUA resulted in statistically significant improvements in the mNIS+7 and the Norfolk QoL-DN total scores, compared to the external placebo control (p<0.001) at Week 35 (Table 2, Figures 1 and 3). The distributions of changes in mNIS+7 and Norfolk QoL-DN scores from baseline to Week 35 by percent of patients in each category are shown in Figure 2 and Figure 4, respectively.
Figure 1: Change from Baseline in mNIS+7 at Week 35 (Comparison of WAINUA Treatment in Study 1 to an External Placebo Control*)
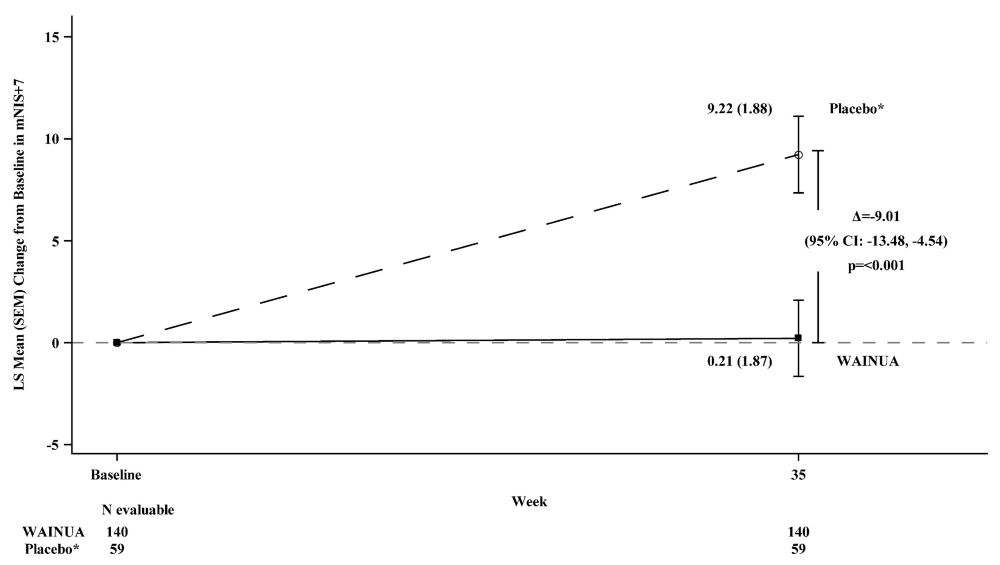
* External placebo group from another randomized controlled trial (NCT01737398).
Figure 2: Histogram of mNIS+7 Change from Baseline at Week 35 (Comparison of WAINUA Treatment in Study 1 to an External Placebo Control*)
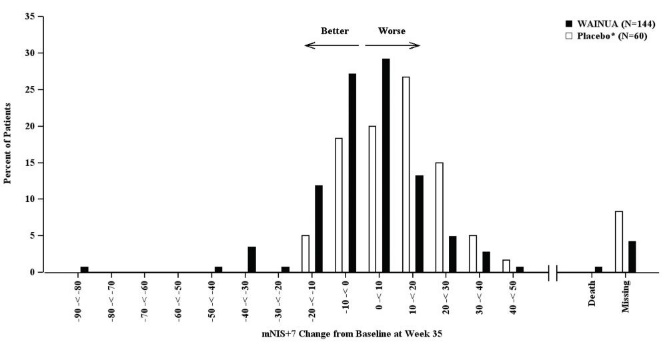
* External placebo group from another randomized controlled trial (NCT01737398).
Figure 3: Change from Baseline in Norfolk QoL-DN Total Score at Week 35 (Comparison of WAINUA Treatment in Study 1 to an External Placebo Control*)
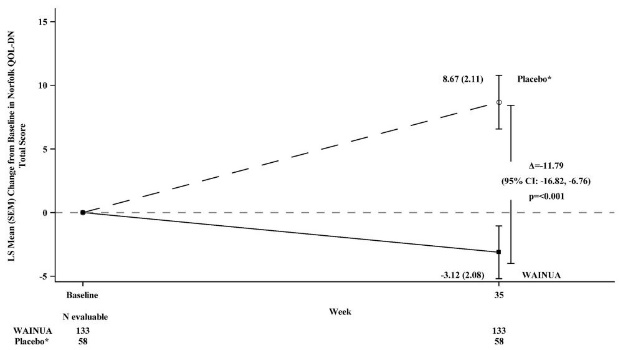
* External placebo group from another randomized controlled trial (NCT01737398).
Figure 4: Histogram of Norfolk QoL-DN Total Score Change from Baseline at Week 35 (Comparison of WAINUA Treatment in Study 1 to an External Placebo Control*)
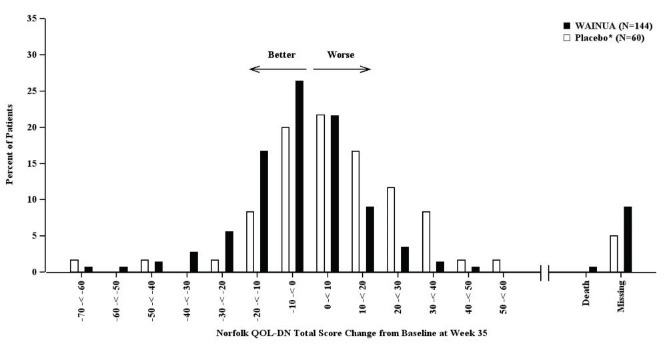
* External placebo group from another randomized controlled trial (NCT01737398).
Patients receiving WAINUA experienced similar improvements relative to those in the external placebo in mNIS+7, and Norfolk QoL-DN score across subgroups including age, sex, race, region, Val30Met variant status, and disease stage.
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Recommended Vitamin A Supplementation
Inform patients that WAINUA treatment leads to a decrease in vitamin A levels measured in the serum. Instruct patients to take the recommended daily allowance of vitamin A. Advise patients to contact their healthcare provider if they experience ocular symptoms suggestive of vitamin A deficiency (e.g., night blindness, dry eyes) and refer them to an ophthalmologist if they develop these symptoms
Pregnancy
Instruct patients that if they are pregnant or plan to become pregnant while taking WAINUA they should inform their healthcare provider. Advise patients of the potential risk to the fetus, including that WAINUA treatment leads to a decrease in serum vitamin A levels
Distributed by: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850
©AstraZeneca 2025
8PATIENT INFORMATION
This Patient Information has been approved by the U.S. Food and Drug Administration Approved: 12/2023
9INSTRUCTIONS FOR USE
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Revised: 12/2025
10PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 0310-9400-01 Rx only
WAINUA™ 45 mg/0.8 mL
(eplontersen) injection
for subcutaneous use
Each WAINUA autoinjector contains 45 mg eplontersen
(equivalent to 47 mg eplontersen sodium) in 0.8 mL of solution, for single-dose only.
Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton protected from light.
Do not freeze. Do not expose to heat.
1 single-dose autoinjector
IONIS™ AstraZeneca





