Brand Name
Sotyktu
Generic Name
Deucravacitinib
View Brand Information FDA approval date: September 09, 2022
Form: Tablet
What is Sotyktu (Deucravacitinib)?
SOTYKTU™ is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. Limitations of Use : SOTYKTU is not recommended for use in combination with other potent immunosuppressants. SOTYKTU is a tyrosine kinase 2 inhibitor indicated for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Limitations of Use : Not recommended for use in combination with other potent immunosuppressants.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Phase 3, Multicenter, Double-blind, Placebo-controlled, Randomized Withdrawal Trial to Evaluate the Efficacy, Safety, and Pharmacokinetics of Deucravacitinib in Children and Adolescents From 5 to Less Than 18 Years of Age With Active Juvenile Psoriatic Arthritis
Summary: The purpose of this study is to evaluate the drug levels, efficacy, and safety of Deucravacitinib (BMS-986165) in pediatric participants with juvenile psoriatic arthritis.
A Multicenter, Randomized, Double-blind, Placebo-controlled Phase 3 Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of Deucravacitinib in Adolescent Participants (12 Years to Less Than 18 Years) With Moderate to Severe Plaque Psoriasis
Summary: The purpose of this study is to evaluate the efficacy, safety, and drug levels of Deucravacitinib (BMS-986165) in adolescent participants with moderate to severe plaque psoriasis
A Phase 3, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy and Safety of Deucravacitinib in Adults With Active Sjögren's Syndrome (POETYK SjS-1)
Summary: The purpose of this study is to assess the safety and efficacy of two doses of Deucravacitinib in adult participants with Active Sjögren's Syndrome.
Related Latest Advances
Brand Information
SOTYKTU (deucravacitinib)
1INDICATIONS AND USAGE
SOTYKTU™ is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Limitations of Use:
SOTYKTU is not recommended for use in combination with other potent immunosuppressants.
2DOSAGE FORMS AND STRENGTHS
Tablets: 6 mg, pink, round, biconvex, laser printed with “BMS 895” and “6 mg” on one side with no content on the other side.
3CONTRAINDICATIONS
SOTYKTU is contraindicated in patients with a history of hypersensitivity reaction to deucravacitinib or to any of the excipients in SOTYKTU
4ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of labeling:
- Infections
- Malignancy including lymphomas
- Laboratory Abnormalities
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of SOTYKTU was evaluated in two placebo- and active-controlled trials (PSO-1 and PSO-2) and an open-label extension trial in which subjects who completed PSO-1 or PSO-2 could enroll
In trials PSO-1 and PSO-2, 1,681 subjects were randomized to receive SOTYKTU 6 mg (840 subjects), placebo (419 subjects), or apremilast 30 mg twice daily (422 subjects). All subjects randomized to placebo switched to SOTYKTU at Week 16. All other subjects remained in their original treatment group until Week 24, at which point subjects could have continued on the same treatment or be switched to SOTYKTU or placebo. The mean age of subjects was 47 years. The majority of subjects were White (87%) and male (67%).
In the 16-week placebo-controlled period of the pooled clinical trials (PSO-1 and PSO-2), discontinuation of therapy due to adverse reactions in subjects who received SOTYKTU was 2.4%, compared to 3.8% for placebo.
Table 1 summarizes the adverse reactions that occurred in at least 1% of subjects in the SOTYKTU group and at a higher rate than the placebo group during the 16-week controlled period.
Adverse reactions that occurred in < 1% of subjects in the SOTYKTU group were herpes zoster.
Specific Adverse Reactions
Exposure adjusted incidence rates are reported for all the adverse reactions presented below.
4.1.1Infections
In the 16-week placebo-controlled period, infections occurred in 29% of the SOTYKTU group (116 events per 100 person-years) compared to 22% of the placebo group (83.7 events per 100 person-years). The majority of infections were non-serious and mild to moderate in severity and did not lead to discontinuation of SOTYKTU.
In the 16-week placebo-controlled period, serious infections were reported in 5 subjects (2.0 per 100 patient-years) treated with SOTYKTU, and 2 subjects (1.6 per 100 patient-years) treated with placebo.
The most common serious infections reported during the 52-week treatment period were pneumonia and COVID-19.
4.1.2Malignancies
During the 0-to-52-week treatment period of the two clinical trials, PSO-1 and PSO-2 (total exposure of 986 patient-years with SOTYKTU), malignancies (excluding non-melanoma skin cancer) were reported in 3 subjects treated with SOTYKTU (0.3 per 100 patient-years), including single cases each of breast cancer, hepatocellular carcinoma, and lymphoma after 24, 32, and 25 weeks of treatment, respectively.
During PSO-1, PSO-2, and the open-label extension trial in which subjects who completed the controlled trials could enroll, a total of 3 subjects (0.1 per 100 patient-years), developed lymphoma while receiving SOTYKTU after 25, 77, and 98 weeks of treatment.
4.1.3Laboratory Abnormalities
Creatine Phosphokinase (CPK)
In the 16-week placebo-controlled period, increased CPK (including Grade 4) was reported in 23 subjects (9.3 per 100 patient-years) treated with SOTYKTU, and 5 subjects (4.1 per 100 patient-years) treated with placebo.
Liver Enzyme Elevations
Events of increases in liver enzymes ≥3 times the ULN were observed in subjects treated with SOTYKTU
- ALT elevations ≥3 times the ULN was reported in 9 subjects (3.6 per 100 patient- years) treated with SOTYKTU, and 2 subjects (1.6 per 100 patient-years) treated with placebo.
- AST elevations ≥3 times the ULN was reported in 13 subjects (5.2 per 100 patient- years) treated with SOTYKTU, and 2 subjects (1.6 per 100 patient-years) treated with placebo.
Decreased Glomerular Filtration Rate (GFR)
In the 16-week placebo-controlled period in subjects who had moderate renal impairment (eGFR 30-59 mL/min) at baseline, decreased GFR was reported in 4 subjects (1.6 per 100 patient-years) treated with SOTYKTU, and 1 subject (0.8 per 100 patient-years) treated with placebo. Two of the deucravacitinib-treated subjects had worsening of baseline proteinuria.
Lipid Elevations
Mean triglycerides increased by 10.3 mg/dL during the 16-week treatment period in subjects treated with SOTYKTU and by 9.1 mg/dL during the 52-week treatment period.
4.1.4Safety Through Week 52
In PSO-1 and PSO-2, the exposure adjusted incidence rate of adverse reactions in subjects treated with SOTYKTU from Week 0 through Week 52 without switching treatment did not increase compared to the rate observed during the first 16 weeks of treatment.
5OVERDOSAGE
There is no experience regarding human overdosage with SOTYKTU. In case of overdose, consider contacting the Poison Help line (1-800-222-1222) for additional overdosage management recommendations.
The extent of deucravacitinib elimination by hemodialysis was small (5.4% of dose per dialysis treatment), and thus hemodialysis for treatment of overdose with SOTYKTU is limited.
6DESCRIPTION
Deucravacitinib is a tyrosine kinase 2 (TYK2) inhibitor and is described chemically as:
6-(cyclopropanecarbonylamido)-4-[2-methoxy-3-(1-methyl-1,2,4-triazol-3-yl)anilino]-N-(trideuteriomethyl)pyridazine-3-carboxamide.
The molecular formula is C
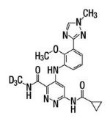
Deucravacitinib is a white to yellow powder. The solubility of deucravacitinib is pH dependent. Solubility decreases with increasing pH.
SOTYKTU (deucravacitinib) tablets are supplied in 6 mg strength for oral administration. Each tablet contains deucravacitinib as the active ingredient and the following inactive ingredients: anhydrous lactose, croscarmellose sodium, hypromellose acetate succinate, magnesium stearate, microcrystalline cellulose, and silicon dioxide. In addition, the film coating Opadry
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide) before starting SOTYKTU therapy and each time the prescription is renewed, as there may be new information they need to know. Advise patients of the potential benefits and risks of SOTYKTU.
Hypersensitivity Reactions
Advise patients to discontinue SOTYKTU and seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions
Infections
Inform patients that SOTYKTU may lower the ability of their immune system to fight infections and to contact their healthcare provider immediately if they develop any signs or symptoms of infection
Inform patients that herpes infections, including serious, may occur with use of SOTYKTU
Malignancies including Lymphomas
Inform patients that SOTYKTU may increase their risk of developing malignancies including lymphomas. Instruct patients to inform their healthcare provider if they have ever had any type of cancer
Rhabdomyolysis
Inform patients that SOTYKTU may increase their risk of developing rhabdomyolysis. Instruct patients to immediately inform their healthcare provider if they develop unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever
Laboratory Abnormalities
Inform patients that SOTYKTU may affect certain lab tests, and that blood tests may be required before and during SOTYKTU treatment
Immunizations
Advise patients that vaccination with live vaccines is not recommended during SOTYKTU treatment. Medications that interact with the immune system may increase the risk of infection following administration of live vaccines. Instruct patients to inform the healthcare practitioner that they are taking SOTYKTU prior to a potential vaccination
Pregnancy
Advise patients to report their pregnancy to Bristol-Myers Squibb Company at 1-800-721-5072
8PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 0003-0895-11
30 Tablets
SOTYKTU
(deucravacitinib)
tablets
6 mg
Rx only
Bristol Myers Squibb





