ConZip
What is ConZip (TraMADol)?
Living with moderate to moderately severe pain can take a toll not just physically, but emotionally and mentally. Whether the pain is from surgery, injury, or a chronic condition, it can interfere with daily activities, sleep, and overall quality of life. ConZip (tramadol hydrochloride) is a medication prescribed to help manage such pain effectively, offering relief when other pain relievers may not be enough.
ConZip belongs to a class of medications called opioid analgesics. However, unlike stronger opioids such as morphine or oxycodone, tramadol works in a dual-action way acting on both opioid receptors and certain brain chemicals that influence how pain signals are perceived. ConZip is an extended-release (ER) formulation designed for around-the-clock pain control, rather than quick, short-term relief. It is a long-established option that bridges the gap between non-opioid pain relievers and more potent opioid medications.
What does ConZip do?
ConZip is prescribed for the management of moderate to moderately severe chronic pain in adults who need continuous, long-term treatment. It is typically used when pain cannot be adequately controlled by over-the-counter painkillers like acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs).
By reducing the brain’s perception of pain and improving comfort levels, ConZip allows patients to perform daily tasks, rest better, and maintain a higher quality of life. It is not intended for use on an “as-needed” basis but rather as a consistent therapy to manage ongoing pain.
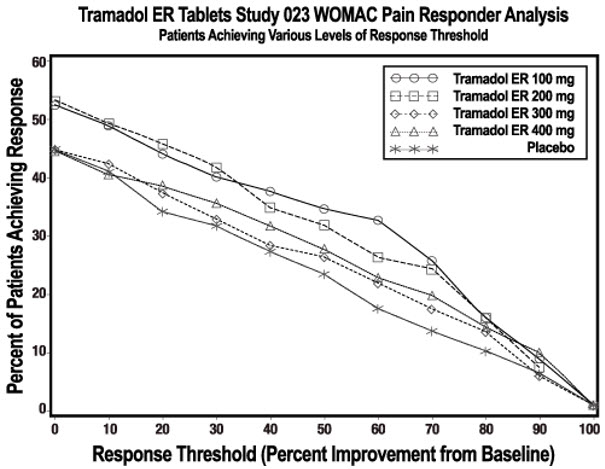
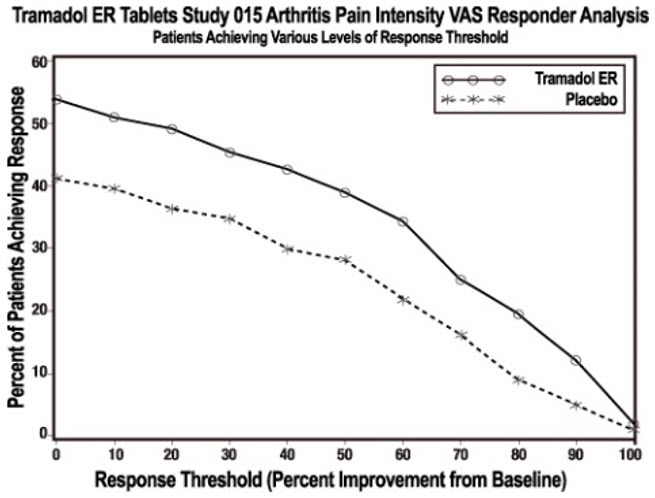
Clinical studies have shown that tramadol effectively relieves pain from neuropathic, musculoskeletal, and post-surgical sources, providing comparable relief to certain other opioids with a potentially lower risk of respiratory depression when used correctly (NIH, 2024). However, it still carries opioid-related risks, and careful monitoring by a healthcare provider is essential.
How does ConZip work?
ConZip’s effectiveness comes from its two mechanisms of action. First, it acts on the mu-opioid receptors in the brain and spinal cord, the same receptors targeted by traditional opioids. This reduces the transmission of pain signals and alters how pain is experienced.
Second, tramadol enhances the activity of serotonin and norepinephrine, two neurotransmitters involved in mood and pain regulation. By increasing their levels, ConZip helps the body’s natural pain-control pathways work more efficiently.
This dual mechanism is clinically important because it not only helps block pain but also modulates how the nervous system processes it. The extended-release formulation of ConZip ensures that the medication is released gradually throughout the day, providing steady pain relief without frequent dosing.
While this makes it effective for chronic pain, it also means patients must take it exactly as prescribed, never crushing or chewing the capsule to avoid dangerous side effects or overdose.
ConZip side effects
Like all opioid medications, ConZip can cause side effects, although not everyone experiences them. Most are mild and improve as the body adjusts to the medication.
Common side effects include:
- Nausea or vomiting
- Dizziness or lightheadedness
- Constipation
- Drowsiness or fatigue
- Headache
- Dry mouth
Serious side effects (less common but important):
- Slowed or difficult breathing
- Confusion, agitation, or hallucinations
- Seizures (especially at high doses or when combined with other medications that affect serotonin)
- Signs of serotonin syndrome such as fever, rapid heartbeat, muscle stiffness, or severe agitation
- Severe allergic reaction (rash, swelling, or difficulty breathing)
Combining tramadol with antidepressants, migraine medications, or certain antibiotics can heighten the risk of serotonin syndrome, a rare but life-threatening reaction, due to its impact on serotonin and norepinephrine levels.
Avoid ConZip if you have severe asthma, a history of seizures/head injury, are taking or recently took MAOIs, or have a hypersensitivity to tramadol or opioids.
Patients should seek immediate medical attention if they experience symptoms like extreme sleepiness, slow breathing, or unresponsiveness which may signal opioid overdose.
Importantly, tramadol has the potential for dependence and misuse. Patients should never increase their dose or share the medication with others. When used under medical supervision, however, it can be a safe and effective option for long-term pain control.
ConZip dosage
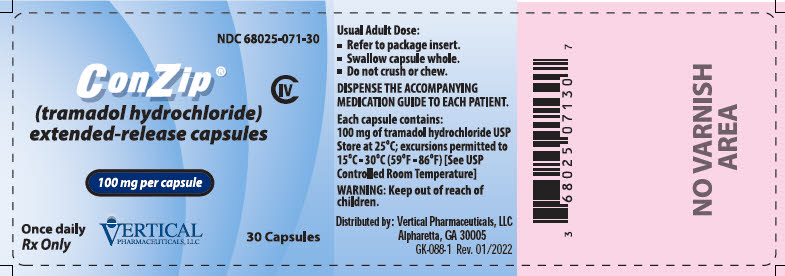
ConZip is an extended-release capsule for once-daily dosing, providing 24-hour pain control. It must be swallowed whole to prevent overdose. Doctors aim for the minimum effective dose.
ConZip doses may need adjustment for patients with liver or kidney disease. Older adults should be closely monitored due to increased risks. Doctors will monitor liver and kidney function, pain, and signs of misuse or dependence.
Does ConZip have a generic version?
Yes. Tramadol hydrochloride extended-release capsules, the generic form of ConZip, are FDA-approved and widely available in the United States. Generic tramadol ER contains the same active ingredient, strength, and dosage form as ConZip and has been shown to be therapeutically equivalent.
Generic versions make treatment more affordable, especially for long-term users. Patients should consult their doctor or pharmacist about generic options. Besides ConZip, tramadol is also available as immediate-release tablets and combination products (e.g., with acetaminophen) under other brand names, with each formulation used for different pain types and durations.
Conclusion
ConZip (tramadol hydrochloride) offers a balanced approach to managing moderate to moderately severe chronic pain. By combining opioid and non-opioid mechanisms, it provides steady, 24-hour pain relief while maintaining a lower risk profile than stronger opioids when used responsibly.
ConZip requires careful use due to potential side effects, dependence, and interactions. Patients must follow provider instructions, avoid unapproved alcohol/sedatives, and report unusual symptoms. When appropriately monitored, ConZip helps manage pain, improve function, and enhance well-being. Open communication with healthcare professionals is vital for safe and successful treatment.
References
- U.S. Food and Drug Administration (FDA). (2024). ConZip (tramadol hydrochloride) prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Tramadol (oral route) drug information. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Tramadol: Uses, side effects, and precautions. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Opioid analgesics and chronic pain management overview. Retrieved from https://www.nih.gov
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- All children younger than 12 years of age
- Postoperative management in children younger than 18 years of age following tonsillectomy and/or adenoidectomy
- Significant respiratory depression
- Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment
- Known or suspected gastrointestinal obstruction, including paralytic ileus
- Hypersensitivity to tramadol (e.g., anaphylaxis)
- Concurrent use of monoamine oxidase inhibitors (MAOIs) or use within the last 14 days
- Addiction, Abuse, and Misuse
- Life-Threatening Respiratory Depression
- Interactions with Benzodiazepines and Other CNS Depressants
- Neonatal Opioid Withdrawal Syndrome
- Ultra-Rapid Metabolism of Tramadol and Other Risk Factors for Life-Threatening Respiratory Depression in Children
- Opioid-Induced Hyperalgesia and Allodynia
- Serotonin Syndrome
- Seizures
- Suicide
- Adrenal Insufficiency
- Severe Hypotension
- Gastrointestinal Adverse Reactions
- Hypersensitivity Reactions
- Withdrawal