Voydeya
What is Voydeya (Danicopan)?
Living with a rare blood disorder like paroxysmal nocturnal hemoglobinuria (PNH) can be physically draining and emotionally overwhelming. Patients often experience fatigue, dark urine, abdominal pain, and anemia, symptoms that stem from the destruction of red blood cells. For many, even with current treatments, these symptoms may not completely disappear. Voydeya (danicopan) offers a new option for patients seeking better control and a higher quality of life.
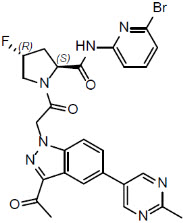
Voydeya is an oral medication approved by the U.S. Food and Drug Administration (FDA) as an add-on therapy for adults with PNH who continue to have clinically significant anemia despite treatment with other complement inhibitors like ravulizumab (Ultomiris) or eculizumab (Soliris). It belongs to a class of drugs known as complement inhibitors, which work by targeting specific parts of the immune system involved in destroying red blood cells.
As a first-in-class Factor D inhibitor, Voydeya represents a significant advancement in PNH management, offering an oral option that complements existing intravenous therapies and provides a more complete level of blood protection.
What does Voydeya do?
Voydeya is used to treat paroxysmal nocturnal hemoglobinuria (PNH), a rare, life-threatening disease where the immune system mistakenly attacks and destroys healthy red blood cells. This process, known as hemolysis, can lead to severe anemia, fatigue, blood clots, and organ damage.
Patients with PNH often receive intravenous complement inhibitors such as Soliris or Ultomiris to block part of the immune system called C5, which helps prevent red blood cell destruction within blood vessels (intravascular hemolysis). However, some patients continue to experience extravascular hemolysis (EVH), a type of red blood cell loss that occurs outside the blood vessels leading to ongoing anemia.
Voydeya is specifically designed to address this unmet need. When used alongside a C5 inhibitor, it helps control both intravascular and extravascular hemolysis, improving red blood cell survival and reducing the need for blood transfusions.
In clinical trials, patients taking Voydeya experienced significant increases in hemoglobin levels and fewer transfusions compared to those receiving standard therapy alone (FDA, 2024). Many reported feeling less fatigued and more energetic, reflecting meaningful improvements in day-to-day functioning.
How does Voydeya work?
Voydeya works by targeting a specific enzyme in the body’s complement system called Factor D. The complement system is part of the immune defense network that helps destroy harmful bacteria and damaged cells. In people with PNH, this system becomes overactive, attacking the body’s own red blood cells instead.
Factor D plays a key role in the alternative pathway of the complement system, one of three routes that can trigger immune attacks on red blood cells. By inhibiting Factor D, Voydeya prevents the activation of this pathway, reducing the destruction of red blood cells both inside and outside blood vessels.
This dual-layer protection helps stabilize hemoglobin levels and decreases symptoms related to anemia, such as tiredness, weakness, and shortness of breath.
Clinically, this mechanism matters because it fills the treatment gap left by other therapies that block only one part of the complement system. By targeting Factor D, Voydeya provides broader, more comprehensive control of PNH-related blood cell damage while maintaining safety and precision.
Voydeya side effects
Like all medications, Voydeya can cause side effects, although not everyone experiences them. Most are mild to moderate and can be managed with medical supervision.
Common side effects may include:
- Headache
- Nausea or vomiting
- Diarrhea
- Fatigue
- Abdominal pain
Serious side effects (less common) include:
- Increased risk of infections, particularly from certain bacteria (e.g., Neisseria meningitidis)
- Allergic reactions, such as rash, swelling, or breathing difficulty
- Liver function abnormalities detected on blood tests
Voydeya, an immune system affecting drug, requires meningococcal vaccination before treatment, with antibiotics sometimes recommended.
Patients must seek immediate medical attention for fever, neck stiffness, confusion, or rash, as these are signs of serious infection. Voydeya is contraindicated in those with active serious infections or hypersensitivity to danicopan or its ingredients.
Close monitoring and prompt reporting of unusual symptoms are crucial for safety with all complement inhibitors.
Voydeya dosage
Voydeya, an oral tablet, is typically combined with other complement inhibitors like Soliris or Ultomiris, offering patients a more convenient alternative to intravenous infusions. Dosing is individualized and adjusted by doctors as needed.
Regular blood tests monitor hemoglobin, liver function, and hemolysis markers for medication safety and effectiveness. Missed doses require doctor’s guidance; never double up to maintain stable complement inhibition.
Older adults and those with mild to moderate liver or kidney issues can generally use Voydeya with careful monitoring, no major dose changes needed.
Does Voydeya have a generic version?
As of 2025, Voydeya (danicopan) does not have a generic version. It is available only as the brand-name product developed and marketed by Alexion Pharmaceuticals, a company known for pioneering complement inhibitor therapies. However, international versions may exist in other markets.
Voydeya, approved by the FDA in 2024, will be patent-protected for years. Generic versions, when available, must meet FDA safety, quality, and effectiveness standards. Patients can explore Alexion’s financial assistance, insurance, or support options for cost management.
Conclusion
Voydeya (danicopan) marks an important step forward for people living with paroxysmal nocturnal hemoglobinuria. By blocking Factor D and working alongside established complement inhibitors, it offers a more complete approach to protecting red blood cells and reducing the burden of anemia.
Voydeya can improve hemoglobin, reduce transfusions, and enhance quality of life for PNH patients who haven’t responded to standard therapy. Close monitoring, side effect management, and infection prevention (including vaccinations) are crucial. Voydeya offers hope for longer, healthier, more energized lives.
References
- U.S. Food and Drug Administration (FDA). (2024). FDA approves Voydeya (danicopan) for adults with PNH and residual anemia. Retrieved from https://www.fda.gov
- Mayo Clinic. (2024). Paroxysmal nocturnal hemoglobinuria (PNH) overview and management. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Danicopan oral: Drug information and safety profile. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Complement inhibitors and Factor D pathway in PNH. Retrieved from https://www.nih.gov
Approved To Treat
Related Clinical Trials
Summary: The primary objective of this study is to evaluate efficacy of danicopan as add-on treatment to ravulizumab or eculizumab as assessed by hemoglobin (Hgb) change from Baseline at Week 12 in pediatric participants with paroxysmal nocturnal hemoglobinuria (PNH) and clinically significant extravascular hemolysis (CS-EVH).
Related Latest Advances
Brand Information
- Complete or update vaccination for encapsulated bacteria specifically,
- Patients receiving VOYDEYA are at increased risk for invasive disease caused by encapsulated bacteria, even if they develop antibodies following vaccination. Monitor patients for early signs and symptoms of serious infections and evaluate immediately if infection is suspected.
- Serious Infections Caused by Encapsulated Bacteria
- Hepatic Enzyme Increases
- Hyperlipidemia