Brand Name
Tudorza Pressair
Generic Name
Aclidinium
View Brand Information FDA approval date: July 01, 2015
Classification: Anticholinergic
Form: Powder
What is Tudorza Pressair (Aclidinium)?
TUDORZA® PRESSAIR® is indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease . TUDORZA PRESSAIR is an anticholinergic indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease .
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Tudorza Pressair (aclidinium bromide)
1INDICATIONS AND USAGE
TUDORZA® PRESSAIR® (aclidinium bromide inhalation powder) is indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD).
2DOSAGE AND ADMINISTRATION
The recommended dose of TUDORZA PRESSAIR is one oral inhalation of 400 mcg, twice daily (morning and evening approximately 12 hours apart).
3DOSAGE FORMS AND STRENGTHS
Inhalation Powder. TUDORZA PRESSAIR is a breath-actuated multi-dose dry powder inhaler metering 400 mcg of aclidinium bromide per actuation.
4CONTRAINDICATIONS
The use of TUDORZA PRESSAIR is contraindicated in the following conditions:
- Severe hypersensitivity to milk proteins
- Hypersensitivity to aclidinium bromide or any of the excipients
5ADVERSE REACTIONS
The following adverse reactions are described in greater detail in other sections:
- Paradoxical bronchospasm
- Worsening of narrow-angle glaucoma
- Worsening of urinary retention
- Immediate hypersensitivity reactions
5.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
5.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of drug TUDORZA PRESSAIR. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
In postmarketing experience with TUDORZA PRESSAIR, immediate hypersensitivity reactions, including anaphylaxis, angioedema (including swelling of the lips, tongue, or throat), urticaria, rash, bronchospasm, or itching have been reported. Additionally, nausea, dysphonia, blurred vision, urinary retention, tachycardia, and stomatitis have been observed.
6DRUG INTERACTIONS
In vitro studies suggest limited potential for CYP450-related metabolic drug interactions, thus no formal drug interaction studies have been performed with TUDORZA PRESSAIR [see .
6.1Sympathomimetics, Methylxanthines, Steroids
In clinical studies, concurrent administration of aclidinium bromide and other drugs commonly used in the treatment of COPD including sympathomimetics (short-acting beta
6.2Anticholinergics
There is a potential for an additive interaction with concomitantly used anticholinergic medications. Therefore, avoid coadministration of TUDORZA PRESSAIR with other anticholinergic-containing drugs as this may lead to an increase in anticholinergic effects.
7DESCRIPTION
TUDORZA PRESSAIR consists of a dry powder formulation of aclidinium bromide for oral inhalation only.
Aclidinium bromide, the active component of TUDORZA PRESSAIR is an anticholinergic with specificity for muscarinic receptors. Aclidinium bromide is a synthetic, quaternary ammonium compound, chemically described as 1-azoniabicyclo[2.2.2]octane, 3-[(hydroxydi-2-thienylacetyl)oxy]-1-(3-phenoxypropyl)-, bromide, (3
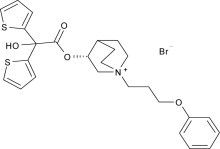
Aclidinium bromide is a white powder with a molecular formula of C
TUDORZA PRESSAIR is a breath-actuated multi-dose dry powder inhaler. Each actuation of TUDORZA PRESSAIR provides a metered dose of 13 mg of the formulation which contains lactose monohydrate (which may contain milk proteins) as the carrier and 400 mcg of aclidinium bromide (equivalent to 343 mcg of aclidinium). This results in delivery of 375 mcg aclidinium bromide (equivalent to 322 mcg of aclidinium) from the mouthpiece, based on
8HOW SUPPLIED/STORAGE AND HANDLING
TUDORZA
The active ingredient is administered using a multi-dose dry powder inhaler, PRESSAIR
Store TUDORZA PRESSAIR in a dry place at 25°C (77°F); excursions permitted to 15-30°C (59-86°F)
The PRESSAIR inhaler should be stored inside the sealed bag and only be opened immediately before use. Throw away the bag.
Throw away (dispose of) the PRESSAIR inhaler after the marking “0” with a red background shows in the middle of the dose indicator, when the device is empty and locks out, or 45 days after the date you opened the sealed bag that the inhaler comes in, whichever comes first.
Keep out of reach of children.
9PATIENT COUNSELING INFORMATION
See FDA-approved Patient Labeling (Patient Information and Instructions for Use)
- Acute Bronchospasm
Instruct patients that TUDORZA PRESSAIR is a twice daily maintenance bronchodilator and should not be used for immediate relief of breathing problems (i.e., as a rescue medication) [see . - Paradoxical Bronchospasm
Inform patients that TUDORZA PRESSAIR can cause paradoxical bronchospasm. Advise patients that if paradoxical bronchospasm occurs, patients should discontinue TUDORZA PRESSAIR [see - Visual Effects
Eye pain or discomfort, blurred vision, visual halos, or colored images in association with red eyes from conjunctival congestion and corneal edema may be signs of acute narrow-angle glaucoma. Inform patients to consult a physician immediately should any of these signs and symptoms develop. Advise patients that miotic eyedrops alone are not considered to be effective treatment [see . - Inform patients that care must be taken not to allow the powder to enter into the eyes as this may cause blurring of vision and pupil dilation.
- Urinary Retention
Difficulty passing urine and dysuria may be symptoms of new or worsening prostatic hyperplasia or bladder outlet obstruction. Patients should be instructed to consult a physician immediately should any of these signs or symptoms develop [see . - Immediate Hypersensitivity Reactions
Inform patients that anaphylaxis, angioedema (including swelling of the lips, tongue, or throat), urticaria, rash, bronchospasm, or itching, may occur after administration of TUDORZA PRESSAIR. Advise patient to immediately discontinue treatment and consult a physician should any of these signs or symptoms develop [see - Instructions for Administering TUDORZA PRESSAIR
It is important for patients to understand how to correctly use TUDORZA PRESSAIR.
Inform patients that if they miss a dose, they should take their next dose at the usual time; they should not take 2 doses at one time.
Manufactured for: Covis Pharma,
Zug, 6300 Switzerland
Under license of ALMIRALL, S.A.
TUDORZA® and the logo are registered trademarks of Almirall, S.A., used under license by Covis Pharma.
PRESSAIR® is a registered trademark of the AstraZeneca group of companies, used under license by Covis Pharma.
© 2022 Covis Pharma
10PRINCIPAL DISPLAY PANEL - 60 Metered Doses Carton

NDC 70515-002-01
R
Tudorza Pressair®
(aclidinium bromide inhalation powder)
400 mcg per actuation
For Oral Inhalation
(aclidinium bromide inhalation powder)
400 mcg per actuation
For Oral Inhalation
60 Metered Doses
COVIS




