Generic Name
Loteprednol Etabonate
Brand Names
Zylet, Eysuvis, Alrex, Inveltys, Lotemax
FDA approval date: March 09, 1998
Classification: Corticosteroid
Form: Ointment, Suspension, Gel
What is Zylet (Loteprednol Etabonate)?
Loteprednol Etabonate Ophthalmic Gel is a corticosteroid indicated for the treatment of post-operative inflammation and pain following ocular surgery. Loteprednol Etabonate Ophthalmic Gel is a corticosteroid indicated for the treatment of postoperative inflammation and pain following ocular surgery.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Zylet (loteprednol etabonate and tobramycin)
1INDICATIONS AND USAGE
ZYLET
Ocular steroids are indicated in inflammatory conditions of the palpebral and bulbar conjunctiva, cornea and anterior segment of the globe such as allergic conjunctivitis, acne rosacea, superficial punctate keratitis, herpes zoster keratitis, iritis, cyclitis, and where the inherent risk of steroid use in certain infective conjunctivitides is accepted to obtain a diminution in edema and inflammation. They are also indicated in chronic anterior uveitis and corneal injury from chemical, radiation or thermal burns, or penetration of foreign bodies.
The use of a combination drug with an anti-infective component is indicated where the risk of superficial ocular infection is high or where there is an expectation that potentially dangerous numbers of bacteria will be present in the eye.
The particular anti-infective drug in this product (tobramycin) is active against the following common bacterial eye pathogens:
Staphylococci, including
2DOSAGE FORMS AND STRENGTHS
Ophthalmic suspension containing 5 mg/mL (0.5%) loteprednol etabonate and 3 mg/mL (0.3%) tobramycin.
3ADVERSE REACTIONS
Adverse reactions have occurred with steroid/anti-infective combination drugs which can be attributed to the steroid component, the anti-infective component, or the combination.
ZYLET
In a 42-day safety study comparing ZYLET to placebo, ocular adverse reactions included injection (approximately 20%) and superficial punctate keratitis (approximately 15%). Increased intraocular pressure was reported in 10% (ZYLET) and 4% (placebo) of subjects. Nine percent (9%) of ZYLET subjects reported burning and stinging upon instillation.
Ocular reactions reported with an incidence less than 4% include vision disorders, discharge, itching, lacrimation disorder, photophobia, corneal deposits, ocular discomfort, eyelid disorder, and other unspecified eye disorders.
The incidence of non-ocular reactions reported in approximately 14% of subjects was headache; all other non-ocular reactions had an incidence of less than 5%.
Loteprednol etabonate ophthalmic suspension 0.2% - 0.5%
Reactions associated with ophthalmic steroids include elevated intraocular pressure, which may be associated with infrequent optic nerve damage, visual acuity and field defects, posterior subcapsular cataract formation, delayed wound healing and secondary ocular infection from pathogens including herpes simplex, and perforation of the globe where there is thinning of the cornea or sclera.
In a summation of controlled, randomized studies of individuals treated for 28 days or longer with loteprednol etabonate, the incidence of significant elevation of intraocular pressure (≥10 mm Hg) was 2% (15/901) among patients receiving loteprednol etabonate, 7% (11/164) among patients receiving 1% prednisolone acetate and 0.5% (3/583) among patients receiving placebo.
Tobramycin ophthalmic solution 0.3%
The most frequent adverse reactions to topical tobramycin are hypersensitivity and localized ocular toxicity, including lid itching and swelling and conjunctival erythema. These reactions occur in less than 4% of patients. Similar reactions may occur with the topical use of other aminoglycoside antibiotics.
Secondary Infection
The development of secondary infection has occurred after use of combinations containing steroids and antimicrobials. Fungal infections of the cornea are particularly prone to develop coincidentally with long-term applications of steroids.
The possibility of fungal invasion must be considered in any persistent corneal ulceration where steroid treatment has been used.
Secondary bacterial ocular infection following suppression of host responses also occurs.
4DESCRIPTION
ZYLET (loteprednol etabonate and tobramycin ophthalmic suspension) is a sterile, multiple dose topical anti-inflammatory corticosteroid and anti-infective combination for ophthalmic use. Both loteprednol etabonate and tobramycin are white to off-white powders. The chemical structures of loteprednol etabonate and tobramycin are shown below.
Loteprednol etabonate:
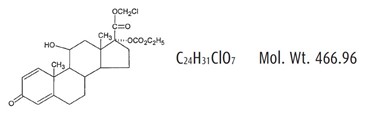
Chemical name: chloromethyl 17α-[(ethoxycarbonyl)oxy]-11
Tobramycin:
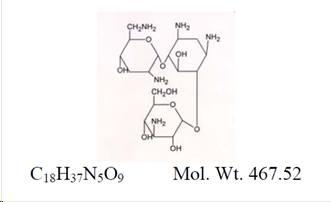
Chemical name:
O-3-Amino-3-deoxy-α-D-glucopyranosyl-(1→4)-O-[2,6-diamino-2,3,6-trideoxy-α-D-ribo-hexopyranosyl-(1→6)]-2-deoxystreptamine
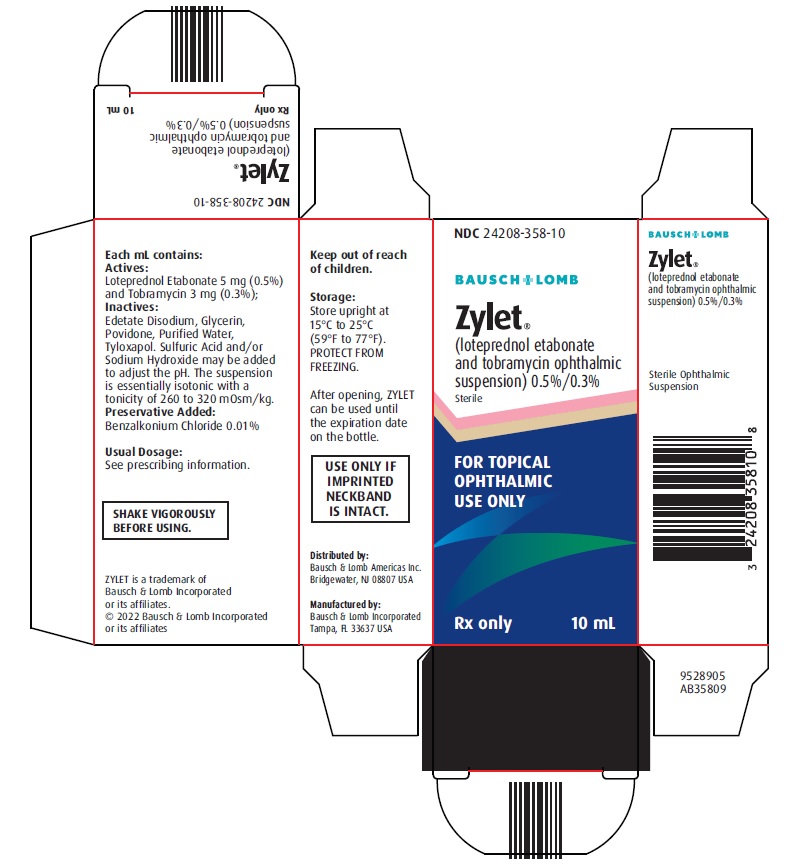
Each mL contains: Actives: Loteprednol Etabonate 5 mg (0.5%) and Tobramycin 3 mg (0.3%). Inactives: Edetate Disodium, Glycerin, Povidone, Purified Water, Tyloxapol, and Benzalkonium Chloride 0.01% (preservative). Sulfuric Acid and/or Sodium Hydroxide may be added to adjust the pH to 5.5 to 6.2. The suspension is essentially isotonic with a tonicity of 260 to 320 mOsm/kg.
5HOW SUPPLIED/STORAGE AND HANDLING
ZYLET (loteprednol etabonate and tobramycin ophthalmic suspension) 0.5%/0.3% is supplied in a white low density polyethylene plastic bottle with a white controlled drop tip and a white polypropylene cap in the following sizes:
NDC 24208-358-05 5 mL fill in a 7.5 mL bottle
NDC 24208-358-10 10 mL fill in a 10 mL bottle
USE ONLY IF IMPRINTED NECKBAND IS INTACT.
Storage: Store upright at 15ºC to 25ºC (59ºF to 77ºF). PROTECT FROM FREEZING. SHAKE VIGOROUSLY BEFORE USING. After opening, ZYLET can be used until the expiration date on the bottle.
6PATIENT COUNSELING INFORMATION
Risk of Contamination
This product is sterile when packaged. Advise patients not to allow the dropper tip to touch any surface, as this may contaminate the suspension.
Risk of Secondary Infection
Advise patients to consult a physician if pain develops, redness, itching or inflammation becomes aggravated.
Contact Lens Wear
As with all ophthalmic preparations containing benzalkonium chloride, advise patients not to wear soft contact lenses when using ZYLET.
Distributed by:
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch & Lomb Incorporated
Tampa, FL 33637 USA
ZYLET and LOTEMAX are trademarks of Bausch & Lomb Incorporated or its affiliates.
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch & Lomb Incorporated
Tampa, FL 33637 USA
ZYLET and LOTEMAX are trademarks of Bausch & Lomb Incorporated or its affiliates.
© 2022 Bausch & Lomb Incorporated or its affiliates
9007709 (FOLDED)
7PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 24208-358-10
BAUSCH + LOMB
Zylet®
(loteprednol etabonate
and tobramycin ophthalmic
suspension) 0.5%/0.3%
Sterile
BAUSCH + LOMB
Zylet®
(loteprednol etabonate
and tobramycin ophthalmic
suspension) 0.5%/0.3%
Sterile
FOR TOPICAL
OPHTHALMIC
USE ONLY
Rx only
OPHTHALMIC
USE ONLY
Rx only
10 mL
9528905