Generic Name
Pyridostigmine
Brand Names
Pryidostigmine, Mestinon, Regonol
FDA approval date: April 06, 1955
Classification: Cholinesterase Inhibitor
Form: Injection, Tablet, Solution
What is Pryidostigmine (Pyridostigmine)?
INDICATION: Pyridostigmine Bromide Oral Solution, USP is useful in the treatment of myasthenia gravis.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Pryidostigmine Bromide (Pyridostigmine Bromide)
1Description
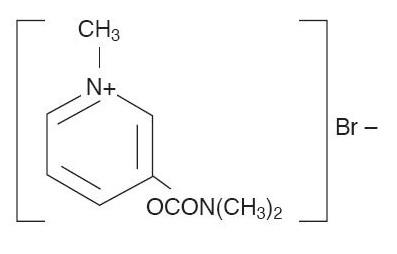
Pyridostigmine bromide oral solution USP is an orally active cholinesterase inhibitor. Chemically, pyridostigmine bromide is 3-hydroxy-1-methylpyridinium bromide dimethylcarbamate. Its structural formula is:
Pyridostigmine bromide syrup is available in the following form: Oral solution containing 60 mg pyridostigmine bromide per teaspoonful in a vehicle containing 5% alcohol, glycerin, lactic acid, sodium benzoate, sorbitol, sucrose, FD&C Red No. 40, FD&C Blue No. 1, raspberry flavor and water.
2CLINICAL PHARMACOLOGY
Pyridostigmine bromide oral solution inhibits the destruction of acetylcholine by cholinesterase and thereby permits freer transmission of nerve impulses across the neuromuscular junction. Pyridostigmine is an analog of neostigmine (Prostigmin®), but differs from it in certain clinically significant respects; for example, pyridostigmine is characterized by a longer duration of action and fewer gastrointestinal side effects.
3INDICATION
Pyridostigmine bromide oral solution USP is useful in the treatment of myasthenia gravis.
4Contraindications
Pyridostigmine bromide oral solution is contraindicated in mechanical intestinal or urinary obstruction, and particular caution should be used in its administration to patients with bronchial asthma. Care should be observed in the use of atropine for counteracting side effects, as discussed below.
5Warnings
Although failure of patients to show clinical improvement may reflect underdosage, it can also be indicative of overdosage. As is true of all cholinergic drugs, overdosage of pyridostigmine bromide oral solution may result in cholinergic crisis, a state characterized by increasing muscle weakness which, through involvement of the muscles of respiration, may lead to death. Myasthenic crisis due to an increase in the severity of the disease is also accompanied by extreme muscle weakness, and thus may be difficult to distinguish from cholinergic crisis on a symptomatic basis. Such differentiation is extremely important, since increases in doses of pyridostigmine bromide oral solution or other drugs of this class in the presence of cholinergic crisis or of a refractory or "insensitive" state could have grave consequences. Osserman and Genkins
Atropine may also be used to abolish or obtund gastrointestinal side effects or other muscarinic reactions; but such use, by masking signs of overdosage, can lead to inadvertent induction of cholinergic crisis.
Usage in Pregnancy
The safety of pyridostigmine bromide oral solution during pregnancy or lactation in humans has not been established. Therefore, use of pyridostigmine bromide oral solution in women who may become pregnant requires weighing the drug's potential benefits against its possible hazards to mother and child.
6Precautions
Pyridostigmine is mainly excreted unchanged by the kidney.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Safety and effectiveness in pediatric patients have not been established.
7Adverse Reactions
The side effects of pyridostigmine bromide oral solution are most commonly related to overdosage and generally are of two varieties, muscarinic and nicotinic. Among those in the former group are nausea, vomiting, diarrhea, abdominal cramps, increased peristalsis, increased salivation, increased bronchial secretions, miosis and diaphoresis. Nicotinic side effects are comprised chiefly of muscle cramps, fasciculation and weakness. Muscarinic side effects can usually be counteracted by atropine, but for reasons shown in the preceding section the expedient is not without danger. As with any compound containing the bromide radical, a skin rash may be seen in an occasional patient. Such reactions usually subside promptly upon discontinuance of the medication.
To report SUSPECTED ADVERSE REACTIONS, contact Athem LLC at 844-369-4671 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
8Dosage and Administration
Pyridostigmine bromide oral solution is available in the following dosage form:
Oral Solution
raspberry-flavored, containing 60 mg pyridostigmine bromide per teaspoonful (5 mL). This form permits accurate dosage adjustment for children and "brittle" myasthenic patients who require fractions of 60 mg doses. It is more easily swallowed, especially in the morning, by patients with bulbar involvement.
raspberry-flavored, containing 60 mg pyridostigmine bromide per teaspoonful (5 mL). This form permits accurate dosage adjustment for children and "brittle" myasthenic patients who require fractions of 60 mg doses. It is more easily swallowed, especially in the morning, by patients with bulbar involvement.
Dosage
The size and frequency of the dosage must be adjusted to the needs of the individual patient.
The size and frequency of the dosage must be adjusted to the needs of the individual patient.
Oral Solution
The average dose is ten 5 mL teaspoonfuls daily, spaced to provide maximum relief when maximum strength is needed. In severe cases, as many as 25 teaspoonfuls a day may be required, while in mild cases one to six teaspoonfuls a day may suffice.
The average dose is ten 5 mL teaspoonfuls daily, spaced to provide maximum relief when maximum strength is needed. In severe cases, as many as 25 teaspoonfuls a day may be required, while in mild cases one to six teaspoonfuls a day may suffice.
Note: For information on a diagnostic test for myasthenia gravis, and for the evaluation and stabilization of therapy, please see product literature on Tensilon® (edrophonium chloride).
9How Supplied
Oral Solution, 60 mg pyridostigmine bromide per teaspoonful (5 mL) and 5% alcohol — bottles of 16 fluid ounces (1 pint) NDC: 73152-028-16
Store pyridostigmine bromide oral solution USP at 25°C (77°F); excursions permitted to 15°C-30°C (59°F-86°F).
Brands mentioned are trademarks of their respective owners.
10References
REFERENCES
Manufactured for:
11Pyridostigmine Bromide Syrup 60mg/5mL Bottle
