Epoprostenol
What is Flolan (Epoprostenol)?
Approved To Treat
Related Clinical Trials
Summary: Pulmonary arterial hypertension (PAH) is a rare, progressive, and potentially fatal disease characterized by increased pulmonary vascular resistance and right ventricular dysfunction. Among the four major molecular pathways involved in PAH pathophysiology-nitric oxide, prostacyclin, activin, and endothelin-1 (ET-1)-the endothelin pathway plays a central role. Endothelin-1 acts on ETA and ETB recep...
Summary: Pulmonary arterial hypertension (PAH) is a progressive disease characterized by vascular remodelling resulting in elevated pressures in the pulmonary artery (PA). This elevated pressure ultimately leads to fulminant right heart failure. Current therapeutic options are limited and are centred around vasodilatory medications such as phosphodiesterase-5 inhibitors and prostacyclin. While these medica...
Summary: Researchers are looking for other ways to treat pulmonary arterial hypertension (PAH). Sotatercept is a study medicine that is designed to treat PAH. A past study, MK-7962-024 (LIGHTRAY) (NCT06664801), learned about the safety and effects of sotatercept in people with PAH. One of the goals of that study was to learn about sotatercept when given at a dose (amount) based on the weight range a person...
Related Latest Advances
Brand Information
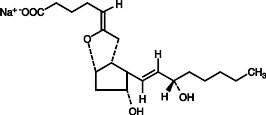
- FLOLAN must be reconstituted only with pH 12 STERILE DILUENT for FLOLAN.
- Reconstituted solutions prepared with pH 12 STERILE DILUENT for FLOLAN do NOT require use with a cold pouch.
- FLOLAN is infused continuously through a permanent indwelling central venous catheter via a small, portable infusion pump. Thus, therapy with FLOLAN requires commitment by the patient to drug reconstitution, drug administration, and care of the permanent central venous catheter. Patients must adhere to sterile technique in preparing the drug and in the care of the catheter, and even brief interruptions in the delivery of FLOLAN may result in rapid symptomatic deterioration. A patient’s decision to receive FLOLAN should be based upon the understanding that there is a high likelihood that therapy with FLOLAN will be needed for prolonged periods, possibly years. Consider the patient's ability to accept and care for a permanent intravenous catheter and infusion pump.
- FLOLAN prepared with pH 12 STERILE DILUENT for FLOLAN must not be used with any preparation or administration materials containing PET or PETG. Only use materials provided by a healthcare provider or pharmacist.
- To adjust infusion rates of FLOLAN only under the direction of a physician.
- To avoid interruptions in drug delivery, the patient should have access to a backup infusion pump and intravenous infusion sets.
- To contact their healthcare providers if any unusual bruising or bleeding develops.
