Generic Name
Glimepiride
Brand Names
Duetact, Pioglitazone
FDA approval date: October 06, 2005
Classification: Sulfonylurea
Form: Tablet
What is Duetact (Glimepiride)?
Glimepiride tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus [see Clinical Studies (1.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Duetact (pioglitazone and glimepiride)
WARNING: CONGESTIVE HEART FAILURE
- Thiazolidinediones, including pioglitazone, which is a component of DUETACT
- After initiation of DUETACT and after dose increases, monitor patients carefully for signs and symptoms of heart failure (e.g., excessive, rapid weight gain, dyspnea, and/or edema). If heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of DUETACT must be considered
- DUETACT is not recommended in patients with symptomatic heart failure
- Initiation of DUETACT in patients with established New York Heart Association (NYHA) Class III or IV heart failure is contraindicated
1INDICATIONS AND USAGE
DUETACT is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus who are already treated with a thiazolidinedione and sulfonylurea or who have inadequate glycemic control on a thiazolidinedione alone or a sulfonylurea alone
Important Limitations of Use
Pioglitazone exerts its antihyperglycemic effect only in the presence of endogenous insulin. DUETACT should not be used to treat type 1 diabetes or diabetic ketoacidosis, as it would not be effective in these settings.
Use caution in patients with liver disease
2DOSAGE FORMS AND STRENGTHS
- 30 mg/2 mg tablet: White to off-white, round, convex tablets, debossed with "4833G" on one side and "30/2" on the other
- 30 mg/4 mg tablet: White to off-white, round, convex tablets, debossed with "4833G" on one side and "30/4" on the other
3CONTRAINDICATIONS
- Initiation in patients with established NYHA Class III or IV heart failure
- Use in patients with known hypersensitivity to pioglitazone, glimepiride or any other component of DUETACT
- Use in patients with known history of an allergic reaction to sulfonamide derivatives.
Reported hypersensitivity reactions with glimepiride include cutaneous eruptions with or without pruritus as well as more serious reactions (e.g., anaphylaxis, angioedema, Stevens-Johnson Syndrome, dyspnea)
4ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Congestive Heart Failure
- Hypoglycemia
- Edema
- Fractures
- Hemolytic Anemia
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The adverse events reported in at least 5% of patients in the controlled 16-week clinical studies between placebo plus a sulfonylurea and pioglitazone (15 mg and 30 mg combined) plus sulfonylurea treatment arms were upper respiratory tract infection (15.5% and 16.6%), accidental injury (8.6% and 3.5%), and combined edema/peripheral edema (2.1% and 7.2%), respectively.
The incidence and type of adverse events reported in at least 5% of patients in any combined treatment group from the 24-week study comparing pioglitazone 30 mg plus a sulfonylurea and pioglitazone 45 mg plus a sulfonylurea are shown in
In US double-blind studies, anemia was reported in ≤2% of patients treated with pioglitazone plus a sulfonylurea
Pioglitazone
Over 8500 patients with type 2 diabetes have been treated with pioglitazone in randomized, double-blind, controlled clinical trials, including 2605 patients with type 2 diabetes and macrovascular disease treated with pioglitazone in the PROactive clinical trial. In these trials, over 6000 patients have been treated with pioglitazone for six months or longer, over 4500 patients have been treated with pioglitazone for one year or longer, and over 3000 patients have been treated with pioglitazone for at least two years.
In six pooled 16- to 26-week placebo-controlled monotherapy and 16- to 24-week add-on combination therapy trials, the incidence of withdrawals due to adverse events was 4.5% for patients treated with pioglitazone and 5.8% for comparator-treated patients. The most common adverse events leading to withdrawal were related to inadequate glycemic control, although the incidence of these events was lower (1.5%) with pioglitazone than with placebo (3.0%).
In the PROactive trial, the incidence of withdrawals due to adverse events was 9.0% for patients treated with pioglitazone and 7.7% for placebo-treated patients. Congestive heart failure was the most common serious adverse event leading to withdrawal occurring in 1.3% of patients treated with pioglitazone and 0.6% of patients treated with placebo.
Common Adverse Events: 16- to 26-Week Monotherapy Trials:
A summary of the incidence and type of common adverse events reported in three pooled 16- to 26-week placebo-controlled monotherapy trials of pioglitazone is provided in
A summary of the overall incidence and types of common adverse events reported in the PROactive trial is provided in
Congestive Heart Failure
A summary of the incidence of adverse events related to congestive heart failure is provided in Table 4 for the 16- to 24-week add-on to sulfonylurea trials, for the 16- to 24-week add-on to insulin trials, and for the 16- to 24-week add-on to metformin trials. None of the events were fatal.
Patients with type 2 diabetes and NYHA class II or early class III congestive heart failure were randomized to receive 24 weeks of double-blind treatment with either pioglitazone at daily doses of 30 mg to 45 mg (n=262) or glyburide at daily doses of 10 mg to 15 mg (n=256). A summary of the incidence of adverse events related to congestive heart failure reported in this study is provided in Table 5.
Congestive heart failure events leading to hospitalization that occurred during the PROactive trial are summarized in Table 6.
Cardiovascular Safety
In the PROactive trial, 5238 patients with type 2 diabetes and a history of macrovascular disease were randomized to pioglitazone (N=2605), force-titrated up to 45 mg daily or placebo (N=2633) in addition to standard of care. Almost all patients (95%) were receiving cardiovascular medications (beta blockers, ACE inhibitors, angiotensin II receptor blockers, calcium channel blockers, nitrates, diuretics, aspirin, statins, and fibrates). At baseline, patients had a mean age of 62 years, mean duration of diabetes of 9.5 years, and mean HbA1c of 8.1%. Mean duration of follow-up was 34.5 months.
The primary objective of this trial was to examine the effect of pioglitazone on mortality and macrovascular morbidity in patients with type 2 diabetes mellitus who were at high risk for macrovascular events. The primary efficacy variable was the time to the first occurrence of any event in a cardiovascular composite endpoint that included all-cause mortality, nonfatal myocardial infarction (MI) including silent MI, stroke, acute coronary syndrome, cardiac intervention including coronary artery bypass grafting or percutaneous intervention, major leg amputation above the ankle, and bypass surgery or revascularization in the leg. A total of 514 (19.7%) patients treated with pioglitazone and 572 (21.7%) placebo-treated patients experienced at least one event from the primary composite endpoint (hazard ratio 0.90; 95% Confidence Interval: 0.80, 1.02; p=0.10).
Although there was no statistically significant difference between pioglitazone and placebo for the three-year incidence of a first event within this composite, there was no increase in mortality or in total macrovascular events with pioglitazone. The number of first occurrences and total individual events contributing to the primary composite endpoint is shown in Table 7.
Weight Gain
Dose-related weight gain occurs when pioglitazone is used alone or in combination with other antidiabetic medications. The mechanism of weight gain is unclear but probably involves a combination of fluid retention and fat accumulation.
Tables 8 and 9 summarize the changes in body weight with pioglitazone and placebo in the 16- to 26-week randomized, double-blind monotherapy and 16- to 24-week combination add-on therapy trials and in the PROactive trial.
Edema
Edema induced from taking pioglitazone is reversible when pioglitazone is discontinued. The edema usually does not require hospitalization unless there is coexisting congestive heart failure. A summary of the frequency and types of edema adverse events occurring in clinical investigations of pioglitazone is provided in Table 10.
Hepatic Effects
There has been no evidence of pioglitazone-induced hepatotoxicity in the pioglitazone-controlled clinical trial database to date. One randomized, double-blind, 3-year trial comparing pioglitazone to glyburide as add-on to metformin and insulin therapy was specifically designed to evaluate the incidence of serum ALT elevation to greater than three times the upper limit of the reference range, measured every eight weeks for the first 48 weeks of the trial then every 12 weeks thereafter. A total of 3/1051 (0.3%) patients treated with pioglitazone and 9/1046 (0.9%) patients treated with glyburide developed ALT values greater than three times the upper limit of the reference range. None of the patients treated with pioglitazone in the pioglitazone-controlled clinical trial database to date have had a serum ALT greater than three times the upper limit of the reference range and a corresponding total bilirubin greater than two times the upper limit of the reference range, a combination predictive of the potential for severe drug-induced liver injury.
Hypoglycemia
In the pioglitazone clinical trials, adverse events of hypoglycemia were reported based on clinical judgment of the investigators and did not require confirmation with fingerstick glucose testing.
In the 16-week add-on to sulfonylurea trial, the incidence of reported hypoglycemia was 3.7% with pioglitazone 30 mg and 0.5% with placebo. In the 16-week add-on to insulin trial, the incidence of reported hypoglycemia was 7.9% with pioglitazone 15 mg, 15.4% with pioglitazone 30 mg, and 4.8% with placebo.
The incidence of reported hypoglycemia was higher with pioglitazone 45 mg compared to pioglitazone 30 mg in both the 24-week add-on to sulfonylurea trial (15.7% versus 13.4%) and in the 24-week add-on to insulin trial (47.8% versus 43.5%).
Three patients in these four trials were hospitalized due to hypoglycemia. All three patients were receiving pioglitazone 30 mg (0.9%) in the 24-week add-on to insulin trial. An additional 14 patients reported severe hypoglycemia (defined as causing considerable interference with patient's usual activities) that did not require hospitalization. These patients were receiving pioglitazone 45 mg in combination with sulfonylurea (N=2) or pioglitazone 30 mg or 45 mg in combination with insulin (N=12).
Urinary Bladder Tumors
Tumors were observed in the urinary bladder of male rats in the two-year carcinogenicity study
Glimepiride
Adverse events that occurred in controlled clinical trials with placebo and glimepiride monotherapy, other than hypoglycemia, included: headache (7.8% and 8.2%), accidental injury (3.4% and 5.8%), flu syndrome (4.4% and 5.4%), nausea (3.4% and 5.0%) and dizziness (2.4% and 5.0%), respectively.
Hypoglycemia
In a randomized, double-blind, placebo-controlled monotherapy trial of 14 weeks duration, patients already on sulfonylurea therapy underwent a 3-week washout period then were randomized to glimepiride 1 mg, 4 mg, 8 mg or placebo. Patients randomized to glimepiride 4 mg or 8 mg underwent forced-titration from an initial dose of 1 mg to these final doses, as tolerated. The overall incidence of possible hypoglycemia (defined by the presence of at least one symptom that the investigator believed might be related to hypoglycemia; a concurrent glucose measurement was not required) was 4% for glimepiride 1 mg, 17% for glimepiride 4 mg, 16% for glimepiride 8 mg and 0% for placebo. All of these events were self-treated.
In a randomized, double-blind, placebo-controlled monotherapy trial of 22 weeks duration, patients received a starting dose of either 1 mg glimepiride or placebo daily. The dose of glimepiride was titrated to a target fasting plasma glucose of 90 −150 mg/dL. Final daily doses of glimepiride were 1, 2, 3, 4, 6 or 8 mg. The overall incidence of possible hypoglycemia (as defined above for the 14-week trial) for glimepiride versus placebo was 19.7% vs. 3.2%. All of these events were self-treated.
Weight Gain
Glimepiride, like all sulfonylureas, can cause weight gain.
Allergic Reactions
In clinical trials, allergic reactions, such as pruritus, erythema, urticaria, and morbilliform or maculopapular eruptions, occurred in less than 1% of glimepiride-treated patients. These may resolve despite continued treatment with glimepiride. There are postmarketing reports of more serious allergic reactions (e.g., dyspnea, hypotension, shock)
Laboratory Tests
Elevated Serum Alanine Aminotransferase (ALT)
In 11 pooled placebo-controlled trials of glimepiride, 1.9% of glimepiride-treated patients and 0.8% of placebo-treated patients developed serum ALT greater than two times the upper limit of the reference range.
Laboratory Abnormalities
Pioglitazone
Hematologic Effects
Pioglitazone may cause decreases in hemoglobin and hematocrit. In placebo-controlled monotherapy trials, mean hemoglobin values declined by 2% to 4% in patients treated with pioglitazone compared with a mean change in hemoglobin of -1% to +1% in placebo-treated patients. These changes primarily occurred within the first 4 to 12 weeks of therapy and remained relatively constant thereafter. These changes may be related to increased plasma volume associated with pioglitazone therapy and are not likely to be associated with any clinically significant hematologic effects.
Creatine Phosphokinase
During protocol-specified measurement of serum creatine phosphokinase (CPK) in pioglitazone clinical trials, an isolated elevation in CPK to greater than 10 times the upper limit of the reference range was noted in nine (0.2%) patients treated with pioglitazone (values of 2150 to 11400 IU/L) and in no comparator-treated patients. Six of these nine patients continued to receive pioglitazone, two patients were noted to have the CPK elevation on the last day of dosing and one patient discontinued pioglitazone due to the elevation. These elevations resolved without any apparent clinical sequelae. The relationship of these events to pioglitazone therapy is unknown.
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of pioglitazone and glimepiride. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Pioglitazone
- New onset or worsening diabetic macular edema with decreased visual acuity
- Fatal and nonfatal hepatic failure
Postmarketing reports of congestive heart failure have been reported in patients treated with pioglitazone, both with and without previously known heart disease and both with and without concomitant insulin administration.
In postmarketing experience, there have been reports of unusually rapid increases in weight and increases in excess of that generally observed in clinical trials. Patients who experience such increases should be assessed for fluid accumulation and volume-related events such as excessive edema and congestive heart failure
Glimepiride
- Serious hypersensitivity reactions, including anaphylaxis, angioedema, and Stevens-Johnson Syndrome
- Hemolytic anemia in patients with and without G6PD deficiency
- Impairment of liver function (e.g. with cholestasis and jaundice), as well as hepatitis, which may progress to liver failure.
- Porphyria cutanea tarda, photosensitivity reactions and allergic vasculitis
- Leukopenia, agranulocytosis, aplastic anemia, and pancytopenia
- Thrombocytopenia (including severe cases with platelet count less than 10,000/mcL) and thrombocytopenic purpura
- Hepatic porphyria reactions and disulfiram-like reactions
- Hyponatremia and syndrome of inappropriate antidiuretic hormone secretion (SIADH), most often in patients who are on other medications or who have medical conditions known to cause hyponatremia or increase release of antidiuretic hormone
5OVERDOSAGE
Pioglitazone
During controlled clinical trials, one case of overdose with pioglitazone was reported. A male patient took 120 mg per day for four days, then 180 mg per day for seven days. The patient denied any clinical symptoms during this period.
In the event of overdosage, appropriate supportive treatment should be initiated according to the patient's clinical signs and symptoms.
Glimepiride
An overdosage of glimepiride, as with other sulfonylureas, can produce severe hypoglycemia. Mild episodes of hypoglycemia can be treated with oral glucose. Severe hypoglycemic reactions constitute medical emergencies requiring immediate treatment. Severe hypoglycemia with coma, seizure, or neurological impairment can be treated with glucagon or intravenous glucose. Continued observation and additional carbohydrate intake may be necessary because hypoglycemia may recur after apparent clinical recovery
6DESCRIPTION
DUETACT tablets are a thiazolidinedione and a sulfonylurea combination product that contains two oral antihyperglycemic agents: pioglitazone and glimepiride. The concomitant use of pioglitazone and a sulfonylurea, the class of drugs that includes glimepiride, has been previously approved based on clinical trials in patients with type 2 diabetes inadequately controlled on a sulfonylurea. Additional efficacy and safety information about pioglitazone and glimepiride monotherapies may be found in the prescribing information for each individual drug.
Pioglitazone is an oral antidiabetic medication.
Pioglitazone [(±)-5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-] thiazolidinedione monohydrochloride contains one asymmetric carbon, and the compound is synthesized and used as the racemic mixture. The two enantiomers of pioglitazone interconvert
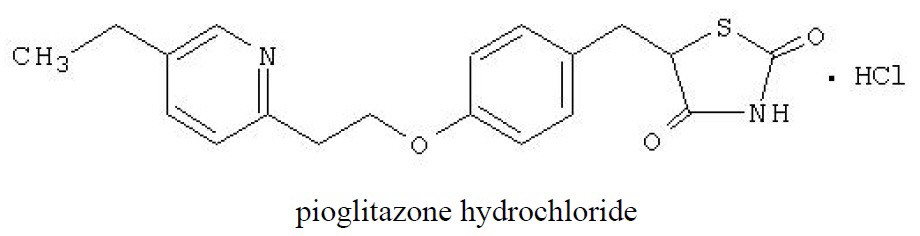
Pioglitazone hydrochloride is an odorless, white crystalline powder that has a molecular formula of C
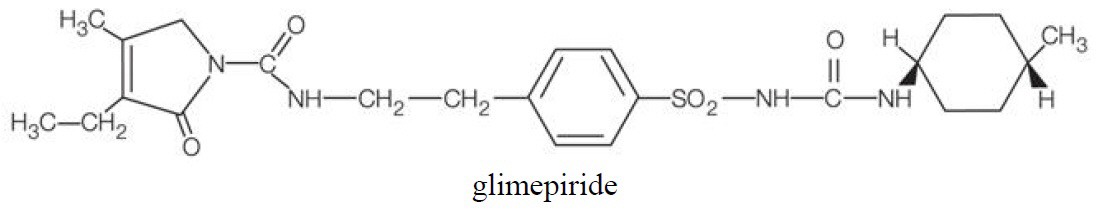
Glimepiride is an oral sulfonylurea chemically identified as 1-[[
DUETACT is available as a tablet for oral administration containing 30 mg pioglitazone (as the base) with 2 mg glimepiride (30 mg/2 mg) or 30 mg pioglitazone (as the base) with 4 mg glimepiride (30 mg/4 mg) formulated with the following excipients: croscarmellose sodium NF, lactose monohydrate NF, magnesium stearate NF, hydroxypropyl cellulose NF, polysorbate 80 NF, and microcrystalline cellulose NF.
7CLINICAL STUDIES
There have been no clinical efficacy studies conducted with DUETACT. However, the efficacy and safety of the separate components have been previously established. The coadministration of pioglitazone and a sulfonylurea, including glimepiride, has been evaluated for efficacy and safety in two clinical studies. These clinical studies established an added benefit of pioglitazone in glycemic control of patients with inadequately controlled type 2 diabetes while on sulfonylurea therapy. Bioequivalence of DUETACT with coadministered pioglitazone and glimepiride tablets was demonstrated at the 30 mg/2 mg and 30 mg/4 mg dosage strengths
Two clinical trials were conducted with pioglitazone in combination with a sulfonylurea. Both studies included patients with type 2 diabetes on any dose of a sulfonylurea, either alone or in combination with another antidiabetic agent. All other antidiabetic agents were withdrawn at least three weeks prior to starting study treatment.
In the first study, 560 patients were randomized to receive 15 mg or 30 mg of pioglitazone or placebo once daily for 16 weeks in addition to their current sulfonylurea regimen. Treatment with pioglitazone as add-on to sulfonylurea produced statistically significant improvements in HbA1c and FGP at endpoint compared to placebo add-on to sulfonylurea
In the second trial, 702 patients were randomized to receive 30 mg or 45 mg of pioglitazone once daily for 24 weeks in addition to their current sulfonylurea regimen. The mean reduction from baseline at Week 24 in HbA1c was 1.6% for the 30 mg dose and 1.7% for the 45 mg dose
The therapeutic effect of pioglitazone in combination with sulfonylurea was observed in patients regardless of the sulfonylurea dose.
8HOW SUPPLIED/STORAGE AND HANDLING
DUETACT is available in 30 mg pioglitazone plus 2 mg glimepiride or 30 mg pioglitazone plus 4 mg glimepiride tablets as follows:
30 mg/2 mg tablet: white to off-white, round, convex tablets, debossed with 4833G on one side and 30/2 on the other, available in:
NDC 64764-302-30 Bottles of 30
NDC 64764-302-90 Bottles of 90
30 mg/4 mg tablet: white to off-white, round, convex tablets, debossed with 4833G on one side and 30/4 on the other, available in:
NDC 64764-304-30 Bottles of 30
NDC 64764-304-90 Bottles of 90
Storage
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Keep container tightly closed and protect from moisture and humidity.
9PATIENT COUNSELING INFORMATION
See
- Inform patients that DUETACT is not recommended for patients with symptoms of heart failure.
- Inform patients that patients with severe heart failure (NYHA Class III or IV) cannot start DUETACT as the risks exceed the benefits in such patients.
- It is important to instruct patients to adhere to dietary instructions and to have blood glucose and glycosylated hemoglobin tested regularly. During periods of stress such as fever, trauma, infection, or surgery, medication requirements may change and patients should be reminded to seek medical advice promptly. Patients should also be informed of the potential risks and advantages of DUETACT and of alternative modes of therapy.
- Tell patients to promptly report any sign of macroscopic hematuria or other symptoms such as dysuria or urinary urgency that develop or increase during treatment as these may be due to bladder cancer.
- Prior to initiation of DUETACT therapy, the risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development should be explained to patients and responsible family members
- Patients who experience an unusually rapid increase in weight or edema or who develop shortness of breath or other symptoms of heart failure while on DUETACT should immediately report these symptoms to a physician.
- Tell patients to promptly stop taking DUETACT and seek immediate medical advice if there is unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or dark urine as these symptoms may be due to hepatotoxicity.
- Inform female patients that treatment with pioglitazone, like other thiazolidinediones may result in an unintended pregnancy in some premenopausal anovulatory females due to its effect on ovulation
- Patients should be told to take a single dose of DUETACT once daily with the first main meal and instructed that any change in dosing should be made only if directed by their physician
Distributed by:
DUETACT is a registered trademark of Takeda Pharmaceutical Company Limited.
© 2025 Takeda Pharmaceuticals America, Inc. All rights reserved.
DTA007 R17
10PRINCIPAL DISPLAY PANEL - 30 mg/2 mg Tablet Bottle Label
NDC 64764-302-30
30 Tablets
30 Tablets
duetact®
pioglitazone 30 mg and
glimepiride 2 mg tablets
pioglitazone 30 mg and
glimepiride 2 mg tablets
Each tablet contains pioglitazone
Dispense with Medication Guide
Takeda
Rx Only
Rx Only

11PRINCIPAL DISPLAY PANEL - 30 mg/4 mg Tablet Bottle Label
NDC 64764-304-30
30 Tablets
30 Tablets
duetact®
pioglitazone 30 mg and
glimepiride 4 mg tablets
pioglitazone 30 mg and
glimepiride 4 mg tablets
Each tablet contains pioglitazone
Dispense with Medication Guide
Takeda
Rx Only
Rx Only
