Generic Name
Adalimumab-AATY
Brand Names
Yuflyma, Adalimumab
FDA approval date: May 23, 2023
Form: Kit
What is Yuflyma (Adalimumab-AATY)?
Adalimumab-aaty is a tumor necrosis factor blocker indicated for: Reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in adult patients with moderately to severely active r heumatoid arthritis.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Yuflyma (adalimumab-aaty)
1DOSAGE FORMS AND STRENGTHS
YUFLYMA is a clear to opalescent, and colorless to pale brown solution available as:
- Auto-injector (YUFLYMA AI)
Injection: 80 mg/0.8 mL in a single-dose auto-injector.
Injection: 40 mg/0.4 mL in a single-dose auto-injector. - Prefilled Syringe with Safety Guard
Injection: 80 mg/0.8 mL in a single-dose prefilled syringe with safety guard.
Injection: 40 mg/0.4 mL in a single-dose prefilled syringe with safety guard. - Prefilled Syringe
Injection: 80 mg/0.8 mL in a single-dose prefilled syringe.
Injection: 40 mg/0.4 mL in a single-dose prefilled syringe.
Injection: 20 mg/0.2 mL in a single-dose prefilled syringe.
Injection: 10 mg/0.1 mL in a single-dose prefilled syringe.
2CONTRAINDICATIONS
None.
3ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Serious Infections
- Malignancies
- Hypersensitivity Reactions
- Hepatitis B Virus Reactivation
- Neurologic Reactions
- Hematological Reactions
- Heart Failure
- Autoimmunity
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reaction with adalimumab was injection site reactions. In placebo-controlled trials, 20% of subjects treated with adalimumab developed injection site reactions (erythema and/or itching, hemorrhage, pain or swelling), compared to 14% of subjects receiving placebo. Most injection site reactions were described as mild and generally did not necessitate drug discontinuation.
The proportion of subjects who discontinued treatment due to adverse reactions during the double-blind, placebo-controlled portion of studies in subjects with RA (i.e., Studies RA-I, RA-II, RA-III and RA-IV) was 7% for subjects taking adalimumab and 4% for placebo-treated subjects. The most common adverse reactions leading to discontinuation of adalimumab in these RA studies were clinical flare reaction (0.7%), rash (0.3%) and pneumonia (0.3%).
3.2Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of adalimumab or of other adalimumab products.
There are two assays that have been used to measure anti-adalimumab antibodies. With the ELISA, antibodies to adalimumab could be detected only when serum adalimumab concentrations were < 2 mcg/mL. The ECL assay can detect anti-adalimumab antibody titers independent of adalimumab concentrations in the serum samples. The incidence of anti-adalimumab antibody (AAA) development in patients treated with adalimumab are presented in Table 2.
3.3Postmarketing Experience
The following adverse reactions have been identified during post-approval use of adalimumab products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to adalimumab products exposure.
Gastrointestinal disorders: Diverticulitis, large bowel perforations including perforations associated with diverticulitis and appendiceal perforations associated with appendicitis, pancreatitis
General disorders and administration site conditions: Pyrexia
Hepato-biliary disorders: Liver failure, hepatitis, autoimmune hepatitis
Immune system disorders: Sarcoidosis
Neoplasms benign, malignant and unspecified (including cysts and polyps): Merkel Cell Carcinoma (neuroendocrine carcinoma of the skin)
Nervous system disorders: Demyelinating disorders (e.g., optic neuritis, Guillain-Barré syndrome), cerebrovascular accident
Respiratory disorders: Interstitial lung disease, including pulmonary fibrosis, pulmonary embolism
Skin reactions: Stevens Johnson Syndrome, cutaneous vasculitis, erythema multiforme, new or worsening psoriasis (all sub-types including pustular and palmoplantar), alopecia, lichenoid skin reaction
Vascular disorders: Systemic vasculitis, deep vein thrombosis
4OVERDOSAGE
Doses up to 10 mg/kg have been administered to patients in clinical trials without evidence of dose-limiting toxicities. In case of overdosage, it is recommended that the patient be monitored for any signs or symptoms of adverse reactions or effects and appropriate symptomatic treatment instituted immediately.
Consider contacting the Poison Help line (1-800-222-1222) or medical toxicologist for additional overdose management recommendations.
5DESCRIPTION
Adalimumab-aaty is a tumor necrosis factor blocker. Adalimumab-aaty is a recombinant human IgG1 monoclonal antibody created using phage display technology resulting in an antibody with human derived heavy and light chain variable regions and human IgG1:k constant regions. Adalimumab-aaty is produced by recombinant DNA technology in a mammalian cell (Chinese Hamster Ovary (CHO)) expression system and is purified by a process that includes specific viral inactivation and removal steps. It consists of 1330 amino acids and has a molecular weight of approximately 148 kilodaltons.
YUFLYMA (adalimumab-aaty) injection is supplied as a sterile, preservative-free solution for subcutaneous administration. The drug product is supplied as either a single-dose, auto-injector (YUFLYMA AI), as a single-dose, 1 mL prefilled syringe with safety guard, or a single-dose, 1 mL prefilled syringe. Enclosed within the auto-injector is a single-dose, 1 mL prefilled syringe. The solution of YUFLYMA is clear to opalescent, and colorless to pale brown, with a pH of about 5.2.
Each 80 mg/0.8 mL prefilled syringe or prefilled syringe with safety guard or auto-injector delivers 0.8 mL (80 mg) of drug product. Each 0.8 mL of YUFLYMA contains adalimumab-aaty (80 mg), acetic acid (0.13 mg), glycine (15.02 mg), polysorbate 80 (0.8 mg), sodium acetate (0.48 mg) and Water for Injection, USP.
Each 40 mg/0.4 mL prefilled syringe or prefilled syringe with safety guard or auto-injector delivers 0.4 mL (40 mg) of drug product. Each 0.4 mL of YUFLYMA contains adalimumab-aaty (40 mg), acetic acid (0.06 mg), glycine (7.51 mg), polysorbate 80 (0.4 mg), sodium acetate (0.24 mg) and Water for Injection, USP.
Each 20 mg/0.2 mL prefilled syringe delivers 0.2 mL (20 mg) of drug product. Each 0.2 mL of YUFLYMA contains adalimumab-aaty (20 mg), acetic acid (0.03 mg), glycine (3.75 mg), polysorbate 80 (0.2 mg), sodium acetate (0.12 mg) and Water for Injection, USP.
Each 10 mg/0.1 mL prefilled syringe delivers 0.1 mL (10 mg) of drug product. Each 0.1 mL of YUFLYMA contains adalimumab-aaty (10 mg), acetic acid (0.02 mg), glycine (1.88 mg), polysorbate 80 (0.1 mg), sodium acetate (0.06 mg) and Water for Injection, USP.
6REFERENCES
- National Cancer Institute. Surveillance, Epidemiology, and End Results Database (SEER) Program. SEER Incidence Crude Rates, 17 Registries, 2000-2007.
7HOW SUPPLIED/STORAGE AND HANDLING
YUFLYMA (adalimumab-aaty) is supplied as a preservative-free, sterile, clear to opalescent, and colorless to pale brown solution for subcutaneous administration. The following packaging configurations are available.
- YUFLYMA AI Carton - 40 mg/0.4 mL (1 Count)
YUFLYMA is supplied in a carton containing two alcohol preps and one dose tray. The dose tray consists of a single-dose prefilled auto-injector, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.4 mL of YUFLYMA. The NDC number is 72606-030-09. - YUFLYMA AI Carton - 40 mg/0.4 mL (2 Count)
YUFLYMA is supplied in a carton containing two alcohol preps and two dose trays. Each dose tray consists of a single-dose prefilled auto-injector, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.4 mL of YUFLYMA. The NDC number is 72606-030-10. - YUFLYMA AI Carton - 40 mg/0.4 mL (4 Count)
YUFLYMA is supplied in a carton containing four alcohol preps and four dose trays. Each dose tray consists of a single-dose prefilled auto-injector, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.4 mL of YUFLYMA. The NDC number is 72606-030-11. - YUFLYMA AI Carton - 40 mg/0.4 mL (6 Count)
YUFLYMA is supplied in a carton containing six alcohol preps and six dose trays. Each dose tray consists of a single-dose prefilled auto-injector, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.4 mL of YUFLYMA. The NDC number is 72606-030-12. - Prefilled Syringe with Safety Guard Carton - 40 mg/0.4 mL (1 Count)
YUFLYMA is supplied in a carton containing two alcohol preps and one dose tray. The dose tray consists of a single-dose prefilled syringe with safety guard, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.4 mL of YUFLYMA. The NDC number is 72606-030-05. - Prefilled Syringe with Safety Guard Carton - 40 mg/0.4 mL (2 Count)
YUFLYMA is supplied in a carton containing two alcohol preps and two dose trays. Each dose tray consists of a single-dose prefilled syringe with safety guard, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.4 mL of YUFLYMA. The NDC number is 72606-030-06. - Prefilled Syringe with Safety Guard Carton - 40 mg/0.4 mL (4 Count)
YUFLYMA is supplied in a carton containing four alcohol preps and four dose trays. Each dose tray consists of a single-dose prefilled syringe with safety guard, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.4 mL of YUFLYMA. The NDC number is 72606-030-07. - Prefilled Syringe with Safety Guard Carton - 40 mg/0.4 mL (6 Count)
YUFLYMA is supplied in a carton containing six alcohol preps and six dose trays. Each dose tray consists of a single-dose prefilled syringe with safety guard, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.4 mL of YUFLYMA. The NDC number is 72606-030-08. - Prefilled Syringe Carton - 40 mg/0.4 mL (1 Count)
YUFLYMA is supplied in a carton containing two alcohol preps and one dose tray. The dose tray consists of a single-dose prefilled syringe, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.4 mL of YUFLYMA. The NDC number is 72606-030-01. - Prefilled Syringe Carton - 40 mg/0.4 mL (2 Count)
YUFLYMA is supplied in a carton containing two alcohol preps and two dose trays. Each dose tray consists of a single-dose prefilled syringe, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.4 mL of YUFLYMA. The NDC number is 72606-030-02. - Prefilled Syringe Carton - 40 mg/0.4 mL (4 Count)
YUFLYMA is supplied in a carton containing four alcohol preps and four dose trays. Each dose tray consists of a single-dose prefilled syringe, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.4 mL of YUFLYMA. The NDC number is 72606-030-03. - Prefilled Syringe Carton - 40 mg/0.4 mL (6 Count)
YUFLYMA is supplied in a carton containing six alcohol preps and six dose trays. Each dose tray consists of a single-dose prefilled syringe, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.4 mL of YUFLYMA. The NDC number is 72606-030-04. - YUFLYMA AI 40 mg/0.4 mL - Plaque Psoriasis Starter Package (4 Count)
YUFLYMA is supplied in a carton containing four alcohol preps and four dose trays (Plaque Psoriasis Starter Package). Each dose tray consists of a single-dose prefilled auto-injector, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.4 mL of YUFLYMA. The NDC number is 72606-030-13. - YUFLYMA AI 40 mg/0.4 mL - Crohn's Disease, Pediatric Crohn's Disease, Ulcerative Colitis or Hidradenitis Suppurativa Starter Package (6 Count)
YUFLYMA is supplied in a carton containing six alcohol preps and six dose trays (Crohn's Disease, Pediatric Crohn’s Disease, Ulcerative Colitis or Hidradenitis Suppurativa Starter Package). Each dose tray consists of a single-dose prefilled auto-injector, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.4 mL of YUFLYMA. The NDC number is 72606-030-14. - YUFLYMA AI Carton - 80 mg/0.8 mL (1 Count)
YUFLYMA is supplied in a carton containing two alcohol preps and one dose tray. The dose tray consists of a single-dose prefilled auto-injector, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 80 mg/0.8 mL of YUFLYMA. The NDC number is 72606-023-04. - YUFLYMA AI Carton - 80 mg/0.8 mL (2 Count)
YUFLYMA is supplied in a carton containing two alcohol preps and two dose trays. Each dose tray consists of a single-dose prefilled auto-injector, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 80 mg/0.8 mL of YUFLYMA. The NDC number is 72606-023-05. - Prefilled Syringe with Safety Guard Carton - 80 mg/0.8 mL (1 Count)
YUFLYMA is supplied in a carton containing two alcohol preps and one dose tray. The dose tray consists of a single-dose prefilled syringe with safety guard, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 80 mg/0.8 mL of YUFLYMA. The NDC number is 72606-023-02. - Prefilled Syringe with Safety Guard Carton - 80 mg/0.8 mL (2 Count)
YUFLYMA is supplied in a carton containing two alcohol preps and two dose trays. Each dose tray consists of a single-dose prefilled syringe with safety guard, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 80 mg/0.8 mL of YUFLYMA. The NDC number is 72606-023-03. - Prefilled Syringe Carton - 80 mg/0.8 mL (1 Count)
YUFLYMA is supplied in a carton containing two alcohol preps and one dose tray. The dose tray consists of a single-dose prefilled syringe, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 80 mg/0.8 mL of YUFLYMA. The NDC number is 72606-023-01. - Prefilled Syringe Carton - 20 mg/0.2 mL (2 Count)
YUFLYMA is supplied in a carton containing two alcohol preps and two dose trays. Each dose tray consists of a single-dose prefilled syringe, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 20 mg/0.2 mL of YUFLYMA. The NDC number is 72606-024-01. - Prefilled Syringe Carton - 10 mg/0.1 mL (2 Count)
YUFLYMA is supplied in a carton containing two alcohol preps and two dose trays. Each dose tray consists of a single-dose prefilled syringe, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 10 mg/0.1 mL of YUFLYMA. The NDC number is 72606-063-01. - YUFLYMA AI 80 mg/0.8 mL and 40 mg/0.4 mL - Plaque Psoriasis Starter Package (3 Count)
YUFLYMA is supplied in a carton containing four alcohol preps and three dose trays (Plaque Psoriasis Starter Package). One dose tray consists of a single-dose prefilled auto-injector, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 80 mg/0.8 mL of YUFLYMA. Each of two other dose trays consists of a single-dose prefilled auto-injector, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.4 mL of YUFLYMA. The NDC number is 72606-046-01. - YUFLYMA AI 80 mg/0.8 mL - Crohn's Disease, Ulcerative Colitis or Hidradenitis Suppurativa Starter Package (3 Count)
YUFLYMA is supplied in a carton containing four alcohol preps and three dose trays (Crohn’s Disease, Ulcerative Colitis or Hidradenitis Suppurativa Starter Package). Each dose tray consists of a single-dose prefilled auto-injector, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 80 mg/0.8 mL of YUFLYMA. The NDC number is 72606-023-07. - YUFLYMA Prefilled Syringe with Safety Guard 80 mg/0.8 mL and 40 mg/0.4 mL - Pediatric Crohn's Disease Starter Package (2 Count)
YUFLYMA is supplied in a carton containing two alcohol preps and two dose trays (Pediatric Crohn’s Disease Starter Package). One dose tray consists of a single-dose prefilled syringe with safety guard, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 80 mg/0.8 mL of YUFLYMA. The other dose tray consists of a single-dose prefilled syringe with safety guard, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 40 mg/0.4 mL of YUFLYMA. The NDC number is 72606-039-01. - YUFLYMA Prefilled Syringe with Safety Guard 80 mg/0.8 mL - Pediatric Crohn's Disease Starter Package (3 Count)
YUFLYMA is supplied in a carton containing four alcohol preps and three dose trays (Pediatric Crohn’s Disease Starter Package). Each dose tray consists of a single-dose prefilled syringe with safety guard, containing a 1 mL prefilled syringe with a fixed thin wall, ½ inch needle, providing 80 mg/0.8 mL of YUFLYMA. The NDC number is 72606-023-08.
8PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
9INSTRUCTIONS FOR USE YUFLYMA®(yoo-fly'-mah) (adalimumab-aaty) injection, for subcutaneous use mg/0.8 mL, 40 mg/0.4 mL Single-Dose Auto-injector
For subcutaneous use only
Read and follow the Instructions for Use that come with your YUFLYMA Auto-injector before you start using it and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or treatment.
Important Information
- Use the Auto-injector
- Ask your doctor how often you will need to give an injection.
- Do not shake the Auto-injector at any time.
- Do not remove the Cap until you are ready to inject.
- Do not share the Auto-injector with anyone.
How to store the Auto-injector
- Store the Auto-injector in a refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the Auto-injector in the original carton until use to protect it from light.
- Do not use an Auto-injector that has been left in direct sunlight.
- Do not freeze the Auto-injector. If the Auto-injector has been frozen, do not use the Auto-injector even if it is thawed.
- If needed, you may store the Auto-injector at room temperature up to 77°F (25°C) for up to 31 days.
- After the Auto-injector has reached room temperature,
- Keep the Auto-injector and all medicines out of the reach of children.
Read Instructions on All Pages Before Using the YUFLYMA Auto-injector
Prepare for Injection
10INSTRUCTIONS FOR USE YUFLYMA®(yoo-fly'-mah) (adalimumab-aaty) injection for subcutaneous use mg/0.8 mL, 40 mg/0.4 mL Single-Dose Prefilled Syringe with Safety Guard
For subcutaneous use only
Read and follow the Instructions for Use that come with your YUFLYMA Prefilled Syringe before you start using it and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or treatment.
Important Information
- Use the Prefilled Syringe with Needle Guard
- Ask your doctor how often you will need to give an injection.
- Do not shake the Prefilled Syringe at any time.
- Do not remove the Cap until you are ready to inject.
- Do not share the Prefilled Syringe with anyone.
- Only use each Prefilled Syringe for one injection.
- Do not pull back on the plunger rod at any time.
How to store the Prefilled Syringe
- Store the Prefilled Syringe in a refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the Prefilled Syringe in the original carton to protect it from light.
- Do not use a Prefilled Syringe that has been left in direct sunlight.
- Do not freeze the Prefilled Syringe. If the Prefilled Syringe has been frozen, do not use the Prefilled Syringe even if it is thawed.
- If needed, you may store the Prefilled Syringe at room temperature up to 77°F (25°C) for up to 31 days.
- After the Prefilled Syringe has reached room temperature,
- Keep the Prefilled Syringe and all medicines out of the reach of children.
Read Instructions on All Pages Before Using the YUFLYMA Prefilled Syringe
Prepare for Injection
11INSTRUCTIONS FOR USE YUFLYMA®(yoo-fly'-mah) (adalimumab-aaty) injection, for subcutaneous use mg/0.8 mL, 40 mg/0.4 mL, 20 mg/0.2 mL, 10 mg/0.1 mL Single-Dose Prefilled Syringe
For subcutaneous use only
Read and follow the Instructions for Use that come with your YUFLYMA Prefilled Syringe before you start using it and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or treatment.
Important Information
- Use the Prefilled Syringe
- Ask your doctor how often you will need to give an injection.
- Do not shake the Prefilled Syringe at any time.
- Do not remove the Cap until you are ready to inject.
- Do not share the Prefilled Syringe with anyone.
- Do not pull back on the plunger rod at any time.
How to store the Prefilled Syringe
- Store the Prefilled Syringe in a refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the Prefilled Syringe in the original carton to protect it from light.
- Do not use a Prefilled Syringe that has been left in direct sunlight.
- Do not freeze the Prefilled Syringe. If the Prefilled Syringe has been frozen,
- If needed, you may store the Prefilled Syringe at room temperature up to 77°F (25°C) for up to 31 days.
- After the Prefilled Syringe has reached room temperature,
- Keep the Prefilled Syringe and all medicines out of the reach of children.
Read Instructions on All Pages Before Using the YUFLYMA Prefilled Syringe
Prepare for Injection
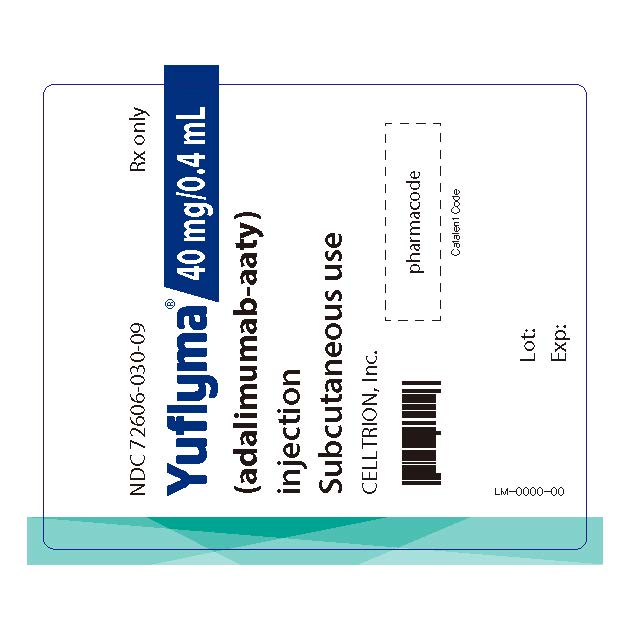
12PRINCIPAL DISPLAY PANEL - 40 mg/0.4 mL Auto-injector Label
NDC 72606-030-09
Yuflyma 40 mg/0.4 mL
Subcutaneous use
CELLTRION Inc.
LM-0000-00
Lot: Exp:
13PRINCIPAL DISPLAY PANEL - 40 mg/0.4 mL Auto-injector Carton
- PRINCIPAL DISPLAY PANEL - 40 mg/0.4 mL Auto-injector Carton 1PK
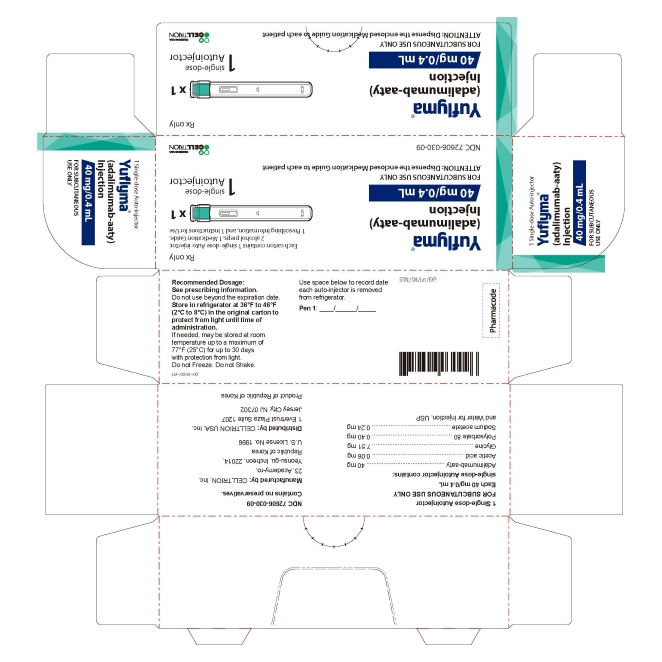
1 Single-dose Auto-injector
Rx only
Yuflyma 40 mg/0.4 mL
FOR SUBCUTANEOUS USE ONLY
1 auto-injector + 2 alcohol preps
NDC 72606-030-09
CELLTRION Inc.
- PRINCIPAL DISPLAY PANEL - 40 mg/0.4 mL Auto-injector Carton 2PK
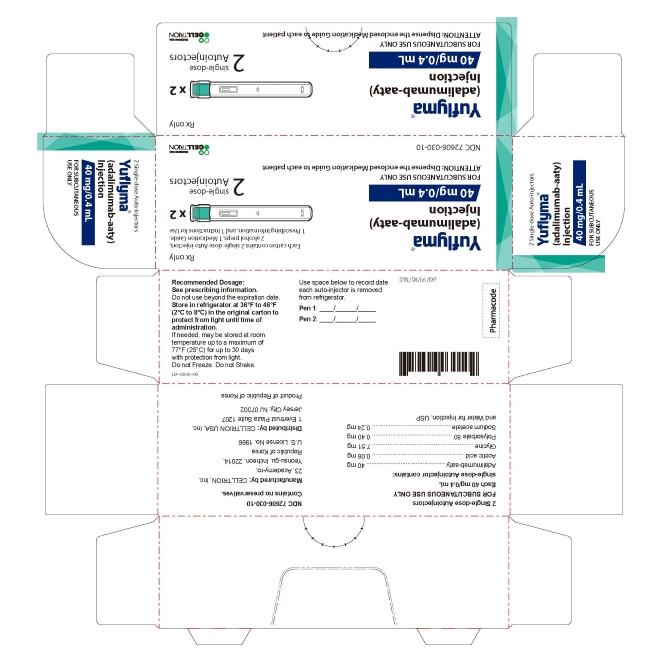
2 Single-dose Auto-injector
Rx only
Yuflyma 40 mg/0.4 mL
adalimumab-aaty
FOR SUBCUTANEOUS USE ONLY
2 auto-injector + 2 alcohol preps
NDC 72606-030-10
CELLTRION Inc.
- PRINCIPAL DISPLAY PANEL - 40 mg/0.4 mL Auto-injector Carton 4PK
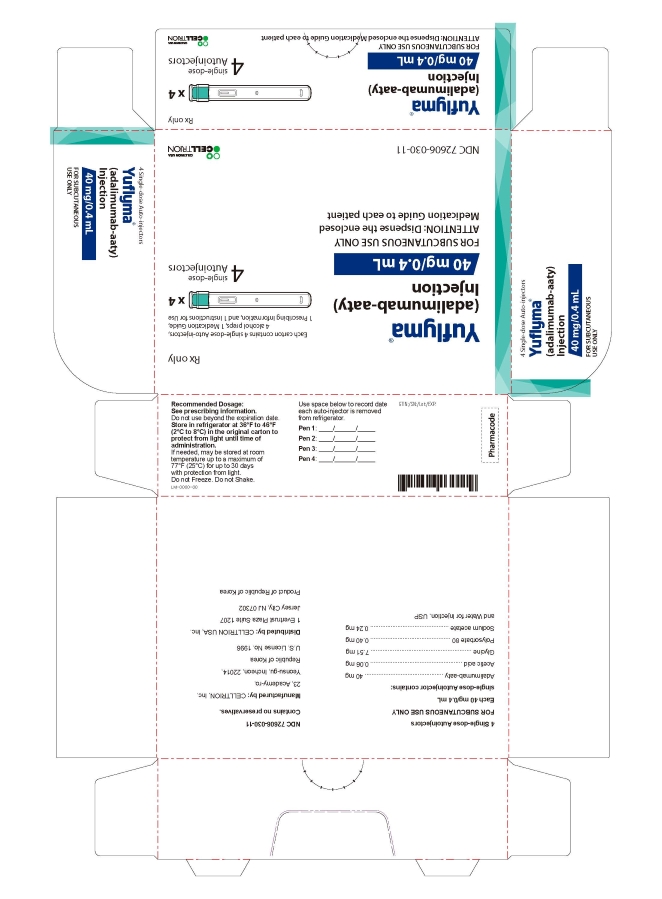
4 Single-dose Auto-injector
Rx only
Yuflyma 40 mg/0.4 mL
adalimumab-aaty
FOR SUBCUTANEOUS USE ONLY
4 auto-injector + 4 alcohol preps
NDC 72606-030-11
CELLTRION Inc.
- PRINCIPAL DISPLAY PANEL - 40 mg/0.4 mL Auto-injector Carton 6PK

6 Single-dose Auto-injector
Rx only
Yuflyma 40 mg/0.4 mL
FOR SUBCUTANEOUS USE ONLY
6 auto-injector + 6 alcohol preps
NDC 72606-030-12
CELLTRION Inc.
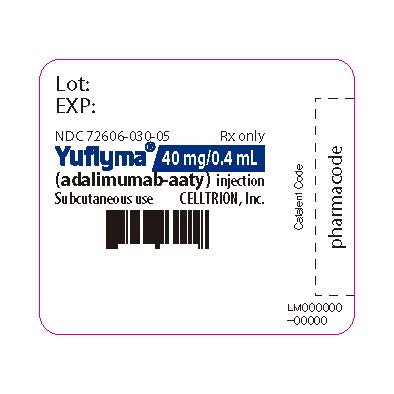
14PRINCIPAL DISPLAY PANEL - 40 mg/0.4 mL Syringe Label- with Guard
Lot:
NDC 72606-030-05
Yuflyma 40 mg/0.4 mL
Subcutaneous use
CELLTRION Inc.
LM000000-00000
15PRINCIPAL DISPLAY PANEL - 40 mg/0.4 mL Syringe Carton - with Guard
- 40 mg/0.4 mL Syringe Carton - with Guard 1PK
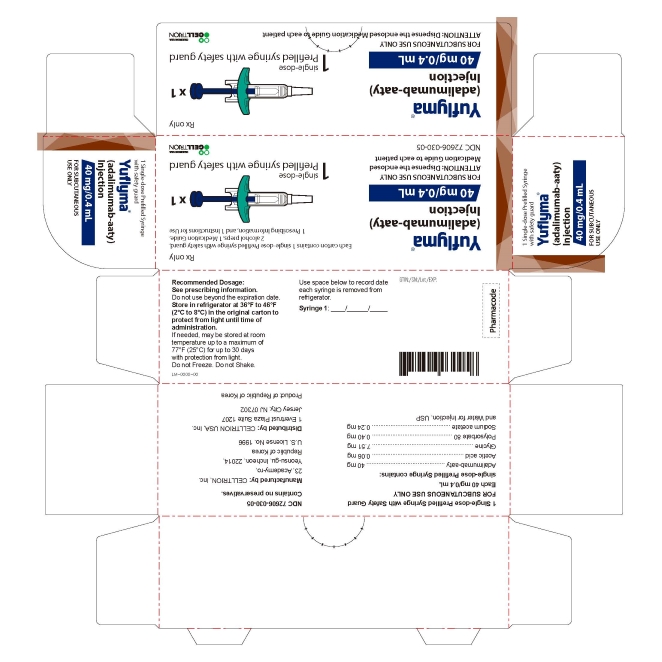
1 Single-dose Prefilled Syringe with Safety Guard
Rx only
Yuflyma 40 mg/0.4 mL
FOR SUBCUTANEOUS USE ONLY
1 prefilled syringe with safety guard + 2 alcohol preps
NDC 72606-030-05
CELLTRION Inc.
- 40 mg/0.4 mL Syringe Carton - with Guard 2PK
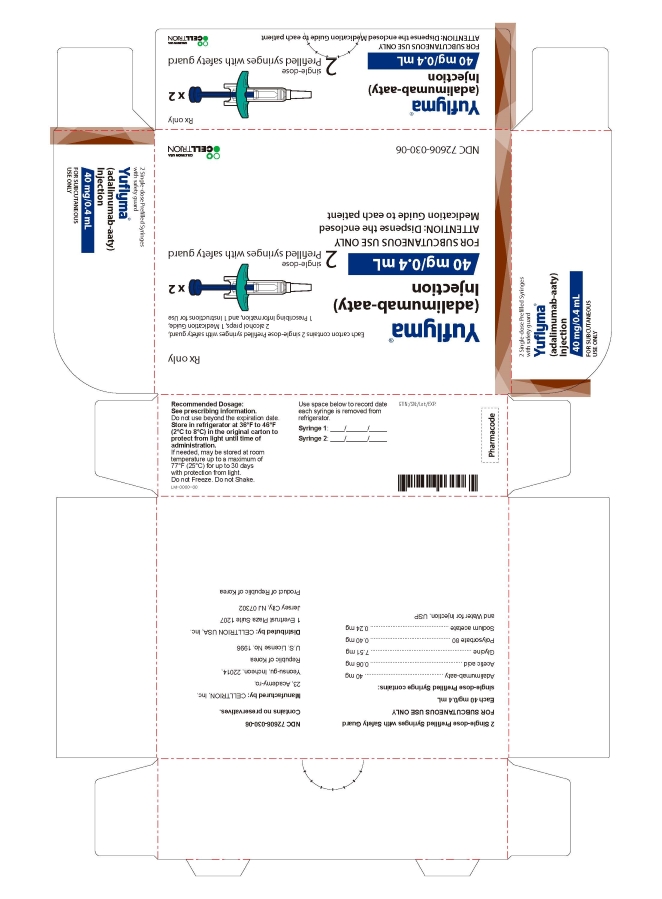
2 Single-dose Prefilled Syringe with Safety Guard
Rx only
Yuflyma 40 mg/0.4 mL
FOR SUBCUTANEOUS USE ONLY
2 prefilled syringe with safety guard + 2 alcohol preps
NDC 72606-030-06
CELLTRION Inc.
- 40 mg/0.4 mL Syringe Carton - with Guard 4PK

4 Single-dose Prefilled Syringe with Safety Guard
Rx only
Yuflyma 40 mg/0.4 mL
FOR SUBCUTANEOUS USE ONLY
4 prefilled syringe with safety guard + 4 alcohol preps
NDC 72606-030-07
CELLTRION Inc.
- 40 mg/0.4 mL Syringe Carton - with Guard 6PK

6 Single-dose Prefilled Syringe with Safety Guard
Rx only
Yuflyma 40 mg/0.4 mL
FOR SUBCUTANEOUS USE ONLY
6 prefilled syringe with safety guard + 6 alcohol preps
NDC 72606-030-08
CELLTRION Inc.
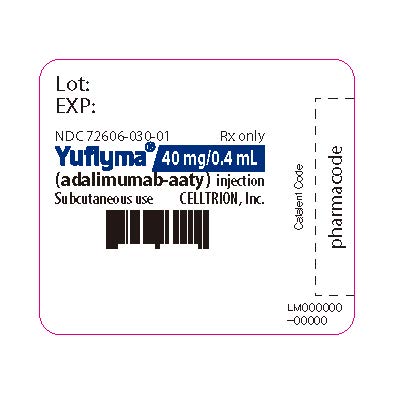
16PRINCIPAL DISPLAY PANEL - 40 mg/0.4 mL Syringe Label
Lot:
NDC 72606-030-01
Yuflyma 40 mg/0.4 mL
Subcutaneous use
CELLTRION Inc.
LM000000-00000
17PRINCIPAL DISPLAY PANEL - 40 mg/0.4 mL Syringe Carton
- 40 mg/0.4 mL Syringe Carton 1PK
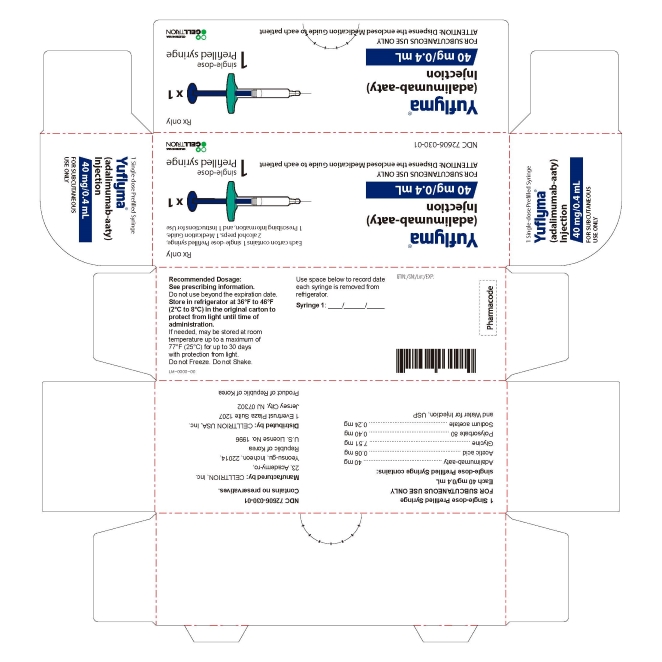
1 Single-dose Prefilled Syringe
FOR SUBCUTANEOUS USE ONLY
1 prefilled syringe + 2 alcohol preps
NDC 72606-030-01
CELLTRION Inc.
- 40 mg/0.4 mL Syringe Carton 2PK

2 Single-dose Prefilled Syringe
FOR SUBCUTANEOUS USE ONLY
2 prefilled syringe + 2 alcohol preps
NDC 72606-030-02
CELLTRION Inc.
- 40 mg/0.4 mL Syringe Carton 4PK

4 Single-dose Prefilled Syringe
FOR SUBCUTANEOUS USE ONLY
4 prefilled syringe + 4 alcohol preps
NDC 72606-030-03
CELLTRION Inc.
- 40 mg/0.4 mL Syringe Carton 6PK

6 Single-dose Prefilled Syringe
FOR SUBCUTANEOUS USE ONLY
6 prefilled syringe + 6 alcohol preps
NDC 72606-030-04
CELLTRION Inc.
18PRINCIPAL DISPLAY PANEL - 40 mg/0.4 mL Auto-injector Carton (Starter Package)
- 40 mg/0.4 mL Auto-injector Carton 4PK - Plaque Psoriasis Starter Package
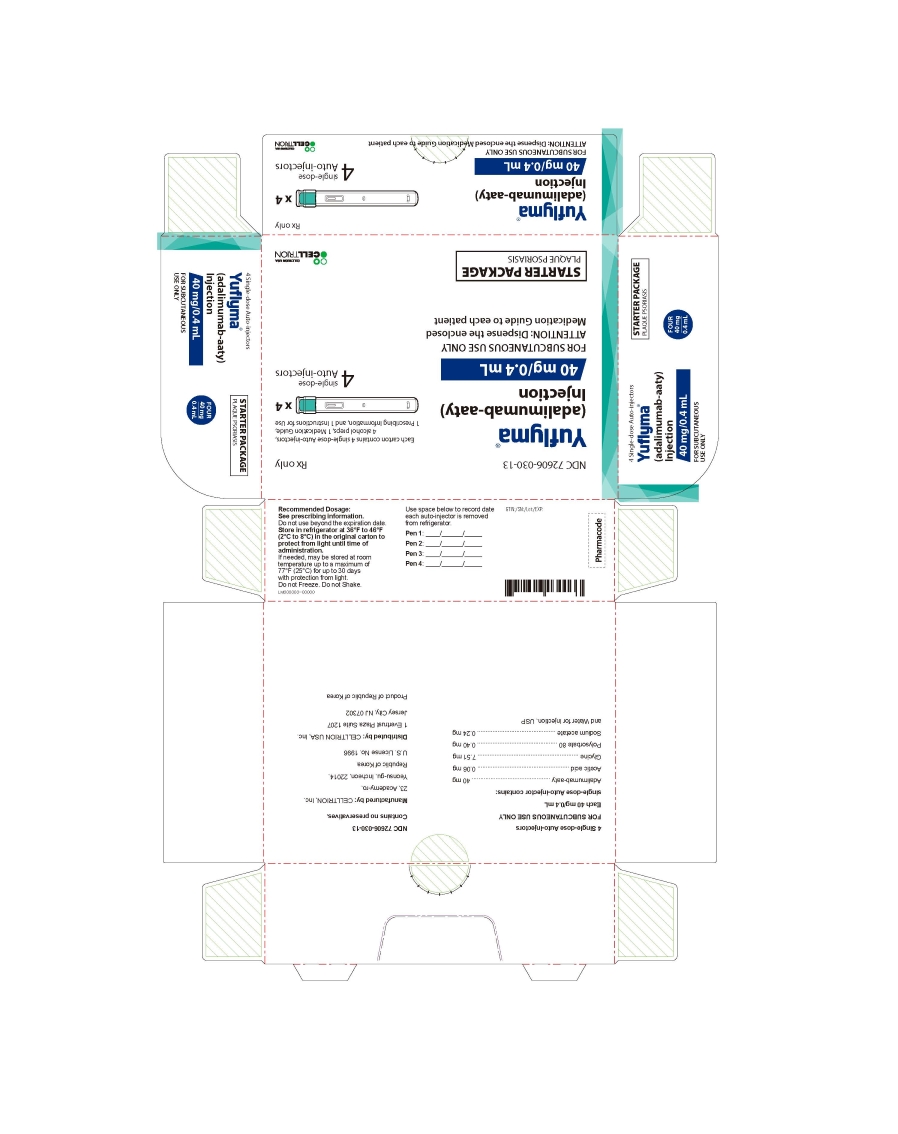
4 Single-dose Auto-injector
Rx only
Yuflyma 40 mg/0.4 mL
adalimumab-aaty
FOR SUBCUTANEOUS USE ONLY
4 auto-injector + 4 alcohol preps
NDC 72606-030-13
CELLTRION Inc.
- 40 mg/0.4 mL Auto-injector Carton 6PK - Crohn's Disease, Pediatric Crohn's Disease, Ulcerative Colitis or Hidradenitis Suppurativa Starter Package

6 Single-dose Auto-injector
Rx only
Yuflyma 40 mg/0.4 mL
adalimumab-aaty
FOR SUBCUTANEOUS USE ONLY
6 auto-injector + 6 alcohol preps
NDC 72606-030-14
CELLTRION Inc.
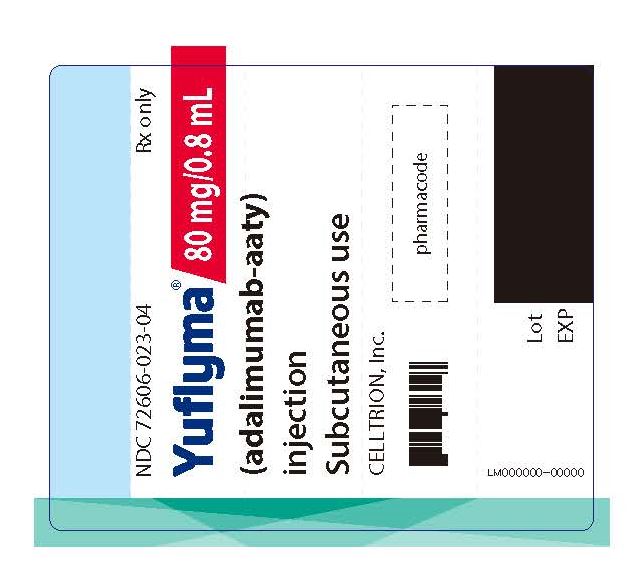
19PRINCIPAL DISPLAY PANEL - 80 mg/0.8 mL Auto-injector Label
NDC 72606-023-04
Yuflyma 80 mg/0.8 mL
Subcutaneous use
CELLTRION Inc.
LM-0000-00
Lot: Exp:
20PRINCIPAL DISPLAY PANEL - 80 mg/0.8 mL Auto-injector Carton
- PRINCIPAL DISPLAY PANEL - 80 mg/0.8 mL Auto-injector Carton 1PK
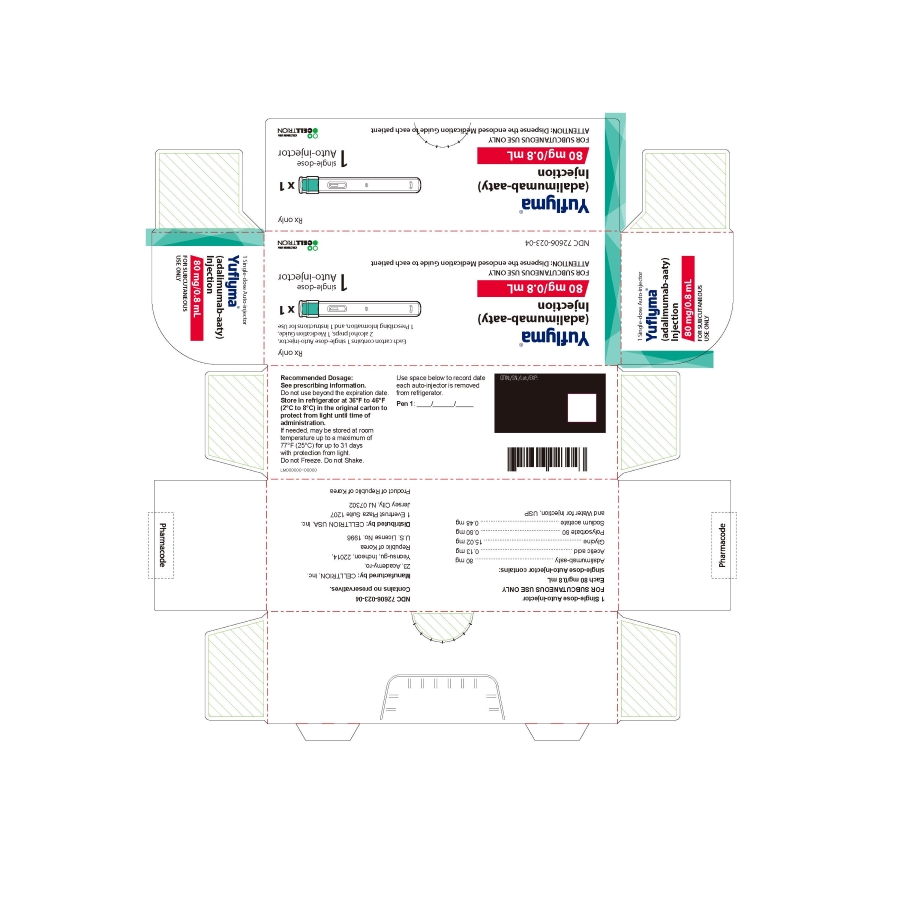
1 Single-dose Auto-injector
Rx only
Yuflyma 80 mg/0.8 mL
FOR SUBCUTANEOUS USE ONLY
1 auto-injector + 2 alcohol preps
NDC 72606-023-04
CELLTRION Inc.
- PRINCIPAL DISPLAY PANEL - 80 mg/0.8 mL Auto-injector Carton 2PK
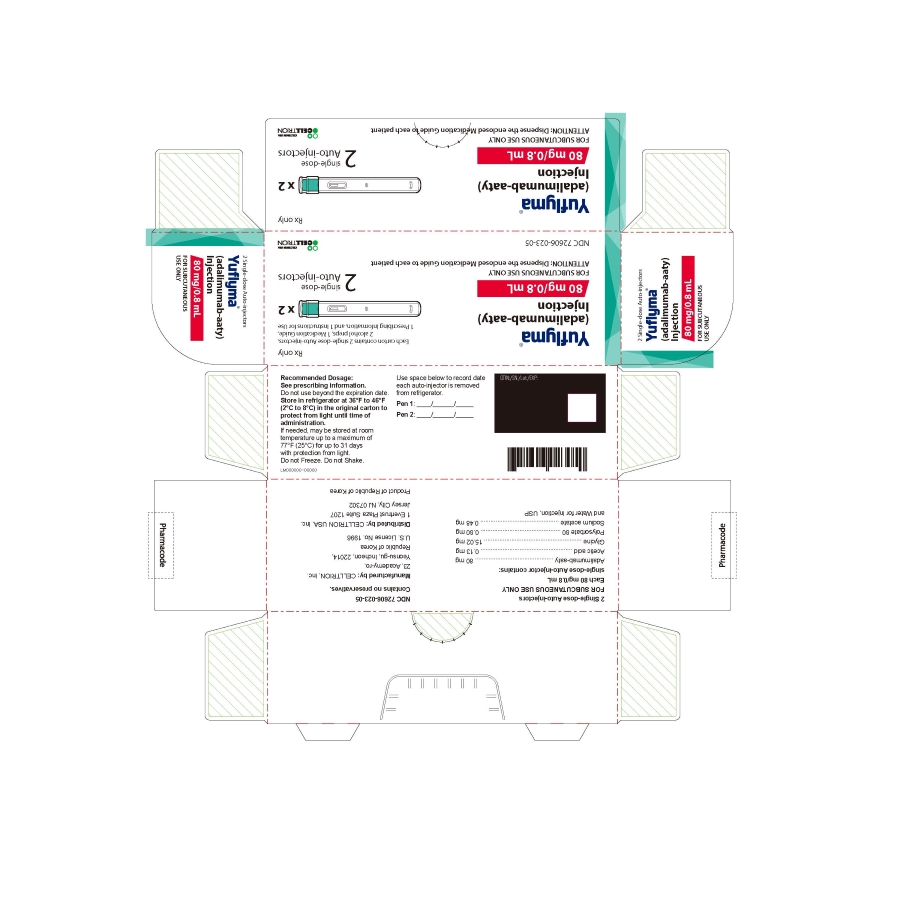
2 Single-dose Auto-injector
Rx only
Yuflyma 80 mg/0.8 mL
adalimumab-aaty
FOR SUBCUTANEOUS USE ONLY
2 auto-injector + 2 alcohol preps
NDC 72606-023-05
CELLTRION Inc.
21PRINCIPAL DISPLAY PANEL - 80 mg/0.8 mL Syringe Label- with Guard
Lot:
NDC 72606-023-02
Yuflyma 80 mg/0.8 mL
Subcutaneous use
CELLTRION Inc.
LM000000-00000

22PRINCIPAL DISPLAY PANEL - 80 mg/0.8 mL Syringe Carton - with Guard
- 80 mg/0.8 mL Syringe Carton - with Guard 1PK

1 Single-dose Prefilled Syringe with Safety Guard
Rx only
Yuflyma 80 mg/0.8 mL
FOR SUBCUTANEOUS USE ONLY
1 prefilled syringe with safety guard + 2 alcohol preps
NDC 72606-023-02
CELLTRION Inc.
- 80 mg/0.8 mL Syringe Carton - with Guard 2PK

2 Single-dose Prefilled Syringe with Safety Guard
Rx only
Yuflyma 80 mg/0.8 mL
FOR SUBCUTANEOUS USE ONLY
2 prefilled syringe with safety guard + 2 alcohol preps
NDC 72606-023-03
CELLTRION Inc.
23PRINCIPAL DISPLAY PANEL - 80 mg/0.8 mL Syringe Label
Lot:
NDC 72606-023-01
Yuflyma 80 mg/0.8 mL
Subcutaneous use
CELLTRION Inc.
LM000000-00000

24PRINCIPAL DISPLAY PANEL - 80 mg/0.8 mL Syringe Carton
- 80 mg/0.8 mL Syringe Carton 1PK

1 Single-dose Prefilled Syringe
FOR SUBCUTANEOUS USE ONLY
1 prefilled syringe + 2 alcohol preps
NDC 72606-023-01
CELLTRION Inc.
25PRINCIPAL DISPLAY PANEL - Kit Carton (STARTER PACKAGE - 80 mg/0.8 mL Auto-injectors)
NDC 72606-023-07
Yuflyma®
80 mg/0.8 mL
FOR SUBCUTANEOUS USE ONLY
ATTENTION: Dispense the enclosed
STARTER PACKAGE
CROHN'S DISEASE, ULCERATIVE COLITIS or HIDRADENITIS SUPPURATIVA
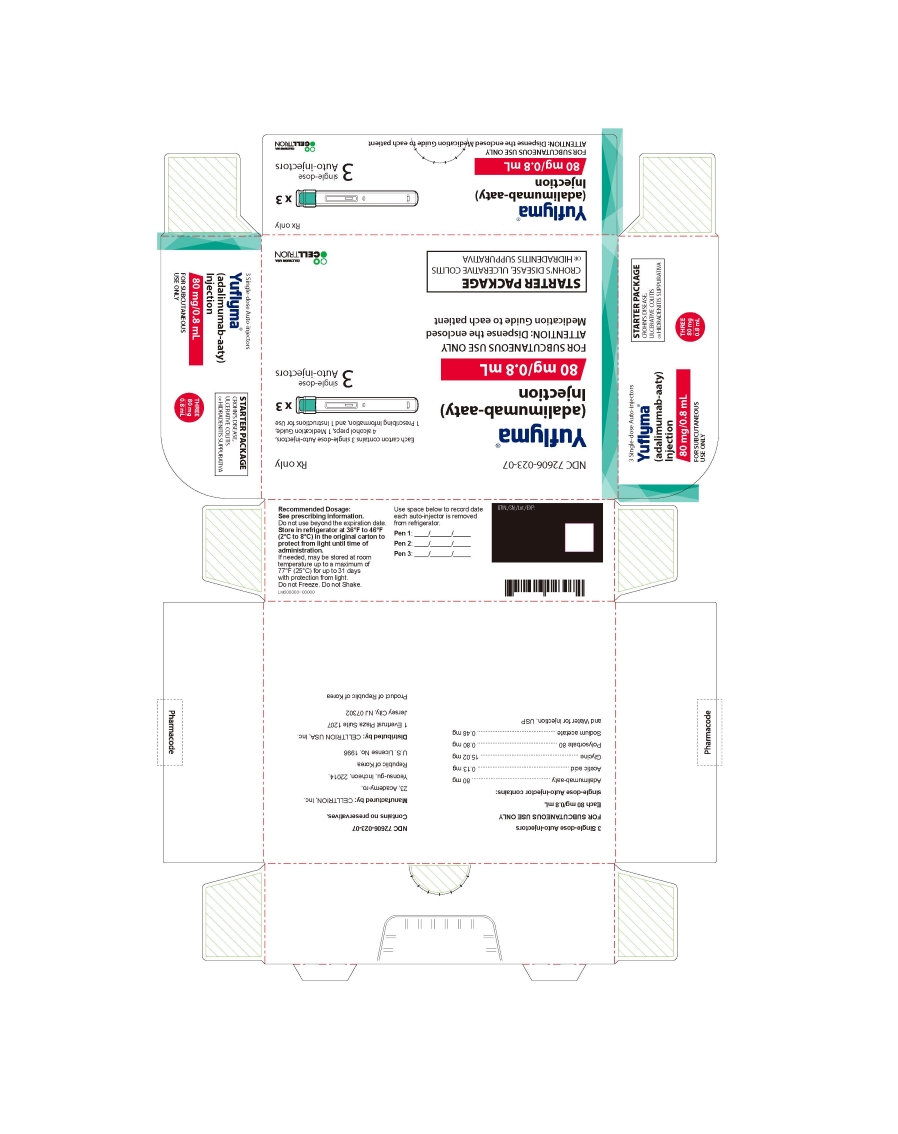
Each carton contains 3 single-dose Auto-injectors.
4 alcohol preps, 1 Medication Guide.
3 single-dose
Auto-injectors
CELLTRION
26PRINCIPAL DISPLAY PANEL - Kit Carton (STARTER PACKAGE - 80 mg/0.8 mL Pre-filled Syringe with Safety Guard)
NDC 72606-023-08
Yuflyma®
80 mg/0.8 mL
FOR SUBCUTANEOUS USE ONLY
ATTENTION: Dispense the enclosed
STARTER PACKAGE
PEDIATRIC CROHN'S DISEASE (≧40 kg (88 lbs))
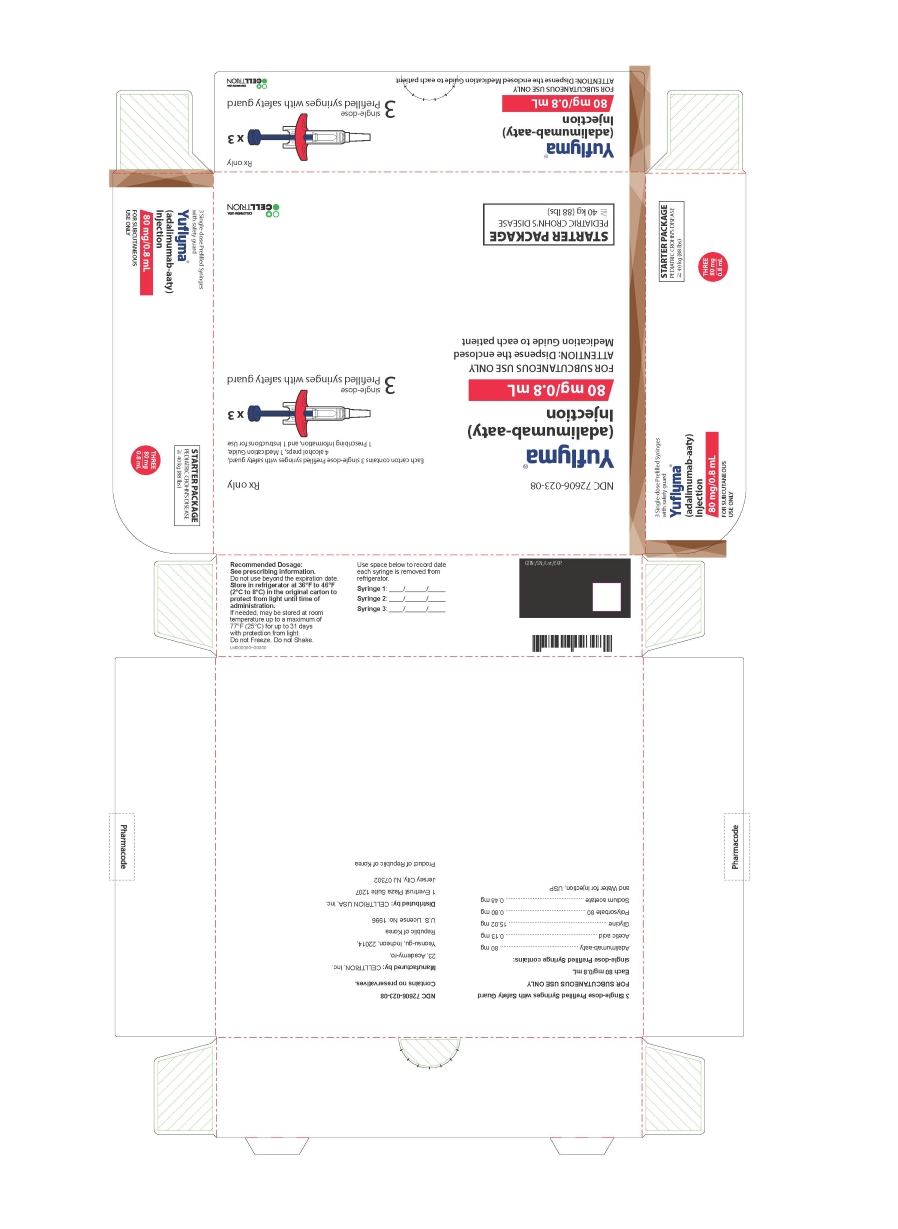
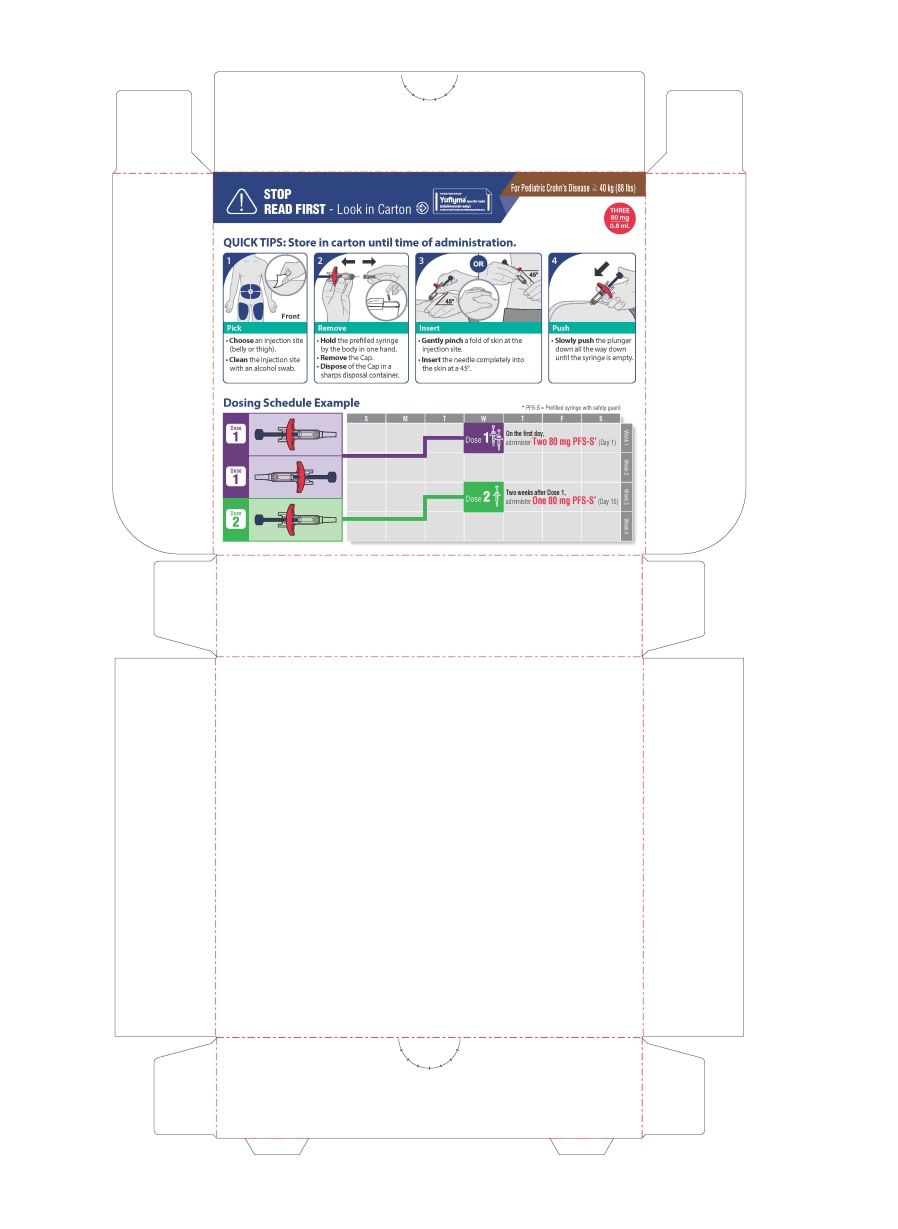
Each carton contains 3 single-dose Prefilled syringes with safety guard.
3 single-dose
Prefilled syringes with safety guard
CELLTRION
27PRINCIPAL DISPLAY PANEL - 20 mg/0.2 mL Syringe Label
Lot:
NDC 72606-024-01
Yuflyma 20 mg/0.2 mL
Subcutaneous use
CELLTRION Inc.
LM000000-00000

28PRINCIPAL DISPLAY PANEL - 20 mg/0.2 mL Syringe Carton
- 20 mg/0.2 mL Syringe Carton 2PK

2 Single-dose Prefilled Syringes
Rx only Yuflyma 20 mg/0.2 mL
adalimumab-aaty
FOR SUBCUTANEOUS USE ONLY
2 prefilled syringes + 2 alcohol preps
NDC 72606-024-01
CELLTRION Inc.
29PRINCIPAL DISPLAY PANEL - Kit Carton (STARTER PACKAGE - 80 mg/0.8 mL and 40 mg/0.4 mL Auto-injectors)
NDC 72606-046-01
Yuflyma®
80 mg/0.8 mL
40 mg/0.4 mL
FOR SUBCUTANEOUS USE ONLY
ATTENTION: Dispense the enclosed
STARTER PACKAGE
PLAQUE PSORIASIS

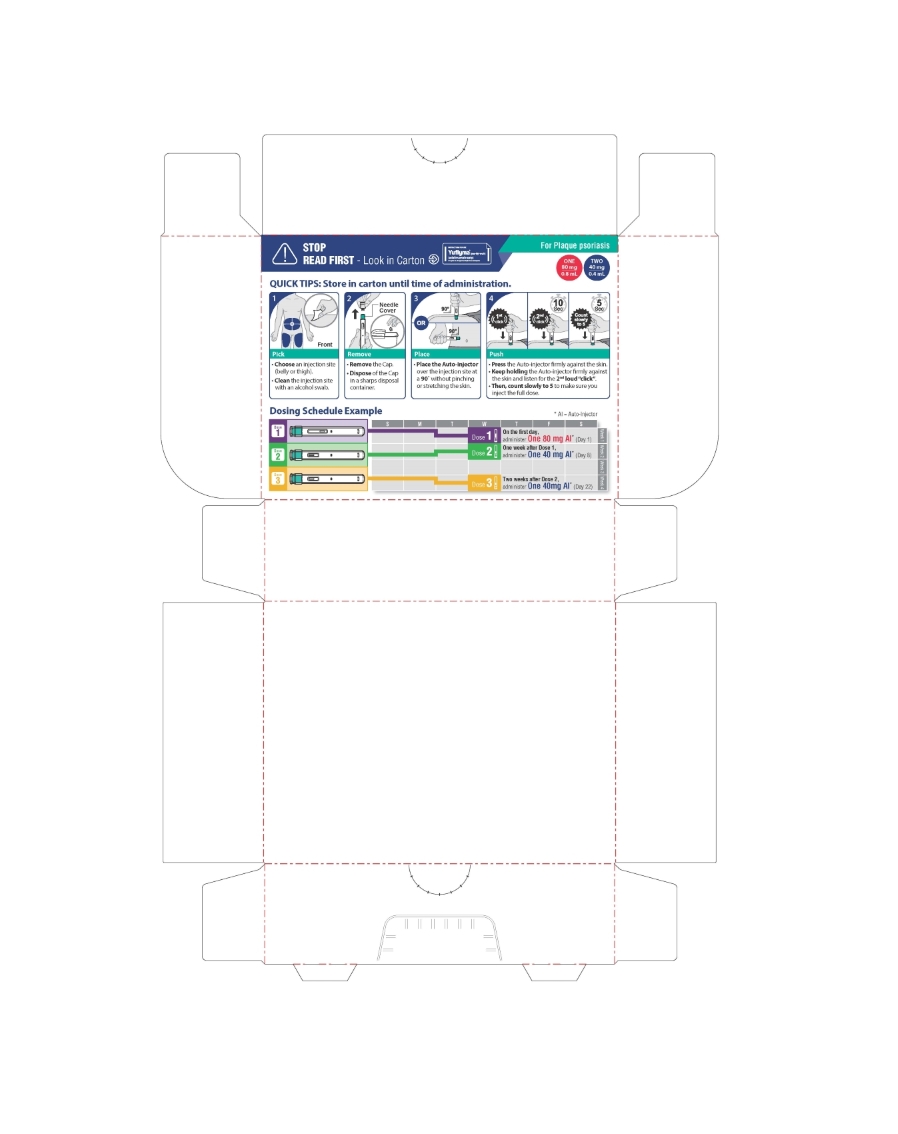
Each carton contains 3 single-dose Auto-injectors.
4 alcohol preps, 1 Medication Guide.
ONE 80 mg/0.8 mL
TWO 40 mg/0.4 mL
3 single-dose
Auto-injectors
CELLTRION
30PRINCIPAL DISPLAY PANEL - Kit Carton (STARTER PACKAGE - 80 mg/0.8 mL and 40 mg/0.4 mL Pre-filled Syringe with Safety Guard)
NDC 72606-039-01
Yuflyma®
80 mg/0.8 mL
40 mg/0.4 mL
FOR SUBCUTANEOUS USE ONLY
ATTENTION: Dispense the enclosed
STARTER PACKAGE
PEDIATRIC CROHN'S DISEASE (17 kg (37 lbs) to <40 kg (88 lbs))

Each carton contains 2 single-dose Pre-filled Syringe with Safety Guard.
2 alcohol preps, 1 Medication Guide.
ONE 80 mg/0.8 mL
ONE 40 mg/0.4 mL
2 single-dose
Pre-filled Syringe with Safety Guard
CELLTRION
31PRINCIPAL DISPLAY PANEL - 10 mg/0.1 mL Syringe Label
Lot:
NDC 72606-063-01
Yuflyma 10 mg/0.1 mL
Subcutaneous use
CELLTRION Inc.
LM000000-00000

32PRINCIPAL DISPLAY PANEL - 10 mg/0.1 mL Syringe Carton
- 10 mg/0.1 mL Syringe Carton 2PK

2 Single-dose Prefilled Syringes
FOR SUBCUTANEOUS USE ONLY
2 prefilled syringes + 2 alcohol preps
NDC 72606-063-01
CELLTRION Inc.