Generic Name
Rosuvastatin
Brand Names
Crestor, Rosuvastain, Ezallor
FDA approval date: July 19, 2016
Classification: HMG-CoA Reductase Inhibitor
Form: Tablet, Capsule
What is Crestor (Rosuvastatin)?
EZALLOR SPRINKLE is indicated: To reduce the risk of major adverse cardiovascular events in adults without established coronary heart disease who are at increased risk of CV disease based on age, high-sensitivity C-reactive protein ≥2 mg/L, and at least one additional CV risk factor., As an adjunct to diet to: o Reduce low-density lipoprotein cholesterol in adults with primary hyperlipidemia. o Reduce LDL-C and slow the progression of atherosclerosis in adults. o Reduce LDL-C in adults and pediatric patients aged 8 years and older with heterozygous familial hypercholesterolemia ., As an adjunct to other LDL-C-lowering therapies, or alone if such treatments are unavailable, to reduce LDL-C in adults and pediatric patients aged 7 years and older with homozygous familial hypercholesterolemia ., As an adjunct to diet for the treatment of adults with: o Primary dysbetalipoproteinemia. o Hypertriglyceridemia. EZALLOR SPRINKLE is an HMG Co-A reductase inhibitor indicated: To reduce the risk of major adverse cardiovascular events in adults without established coronary heart disease who are at increased risk of CV disease based on age, high- sensitivity C-reactive protein ≥2 mg/L, and at least one additional CV risk factor. As an adjunct to diet to: reduce LDL-C in adults with primary hyperlipidemia. reduce LDL-C and slow the progression of atherosclerosis in adults. reduce LDL-C in adults and pediatric patients aged 8 years and older with heterozygous familial hypercholesterolemia . As an adjunct to other LDL-C-lowering therapies, or alone if such treatments are unavailable, to reduce LDL-C in adults and pediatric patients aged 7 years and older with homozygous familial hypercholesterolemia . As an adjunct to diet for the treatment of adults with: o Primary dysbetalipoproteinemia. o Hypertriglyceridemia.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Crestor (Rosuvastatin)
1INDICATIONS AND USAGE
CRESTOR is indicated:
- To reduce the risk of major adverse cardiovascular (CV) events (CV death, nonfatal myocardial infarction, nonfatal stroke, or an arterial revascularization procedure) in adults without established coronary heart disease who are at increased risk of CV disease based on age, high-sensitivity C-reactive protein (hsCRP) ≥2 mg/L, and at least one additional CV risk factor.
- As an adjunct to diet to:
- As an adjunct to other LDL-C-lowering therapies, or alone if such treatments are unavailable, to reduce LDL-C in adults and pediatric patients aged 7 years and older with homozygous familial hypercholesterolemia (HoFH).
- As an adjunct to diet for the treatment of adults with:
2DOSAGE FORMS AND STRENGTHS
CRESTOR tablets:
- 5 mg of rosuvastatin: yellow, round, biconvex, coated tablets. Debossed “ZD4522” and “5” on one side of the tablet.
- 10 mg of rosuvastatin: pink, round, biconvex, coated tablets. Debossed “ZD4522” and “10” on one side of the tablet.
- 20 mg of rosuvastatin: pink, round, biconvex, coated tablets. Debossed “ZD4522” and “20” on one side of the tablet.
- 40 mg of rosuvastatin: pink, oval, biconvex, coated tablets. Debossed “ZD4522” on one side and “40” on the other side of the tablet.
3CONTRAINDICATIONS
CRESTOR is contraindicated in the following conditions:
- Acute liver failure or decompensated cirrhosis
- Hypersensitivity to rosuvastatin or any excipients in CRESTOR. Hypersensitivity reactions including rash, pruritus, urticaria, and angioedema have been reported with CRESTOR
4ADVERSE REACTIONS
The following important adverse reactions are described below and elsewhere in the labeling:
Myopathy and Rhabdomyolysis
Immune-Mediated Necrotizing Myopathy
Hepatic Dysfunction
Proteinuria and Hematuria
Increases in HbA1c and Fasting Serum Glucose Levels
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adverse reactions reported in ≥2% of patients in placebo-controlled clinical studies and at a rate greater than placebo are shown in Table 2. These studies had a treatment duration of up to 12 weeks.
Other adverse reactions reported in clinical studies were abdominal pain, dizziness, hypersensitivity (including rash, pruritus, urticaria, and angioedema) and pancreatitis. The following laboratory abnormalities have also been reported: dipstick-positive proteinuria and microscopic hematuria; elevated creatine phosphokinase, transaminases, glucose, glutamyl transpeptidase, alkaline phosphatase, and bilirubin; and thyroid function abnormalities.
In the METEOR study, patients were treated with CRESTOR 40 mg (n=700) or placebo (n=281) with a mean treatment duration of 1.7 years. Adverse reactions reported in ≥2% of patients and at a rate greater than placebo are shown in Table 3.
In the JUPITER study, patients were treated with CRESTOR 20 mg (n=8,901) or placebo (n=8,901) for a mean duration of 2 years. In JUPITER, there was a significantly higher frequency of diabetes mellitus reported in patients taking CRESTOR (2.8%) versus patients taking placebo (2.3%). Mean HbA1c was significantly increased by 0.1% in CRESTOR-treated patients compared to placebo-treated patients. The number of patients with a HbA1c >6.5% at the end of the trial was significantly higher in CRESTOR-treated versus placebo-treated patients
Adverse reactions reported in ≥2% of patients and at a rate greater than placebo are shown in Table 4.
Pediatric Patients with HeFH
In a 12‑week controlled study in pediatric patients 10 to 17 years of age with HeFH with CRESTOR 5 mg to 20 mg daily
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of CRESTOR. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood Disorders: thrombocytopenia
Hepatobiliary Disorders: hepatitis, jaundice, fatal and non-fatal hepatic failure
Musculoskeletal Disorders: arthralgia, rare reports of immune-mediated necrotizing myopathy associated with statin use
Nervous System Disorders: peripheral neuropathy, rare postmarketing reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, and confusion) associated with the use of all statins. The reports are generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks). There have been rare reports of new-onset or exacerbation of myasthenia gravis, including ocular myasthenia, and reports of recurrence when the same or a different statin was administered.
Psychiatric Disorders: depression, sleep disorders (including insomnia and nightmares)
Reproductive System and Breast Disorders: gynecomastia
Respiratory Disorders: interstitial lung disease
Skin and Subcutaneous Tissue Disorders: drug reaction with eosinophilia and systemic symptoms (DRESS), lichenoid drug eruption
5OVERDOSAGE
No specific antidotes for CRESTOR are known. Hemodialysis does not significantly enhance clearance of rosuvastatin. In the event of overdose, consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
6DESCRIPTION
CRESTOR (rosuvastatin) is a 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA)-reductase inhibitor.
The chemical name for rosuvastatin calcium is bis[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino] pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhept-6-enoic acid] calcium salt with the following structural formula:
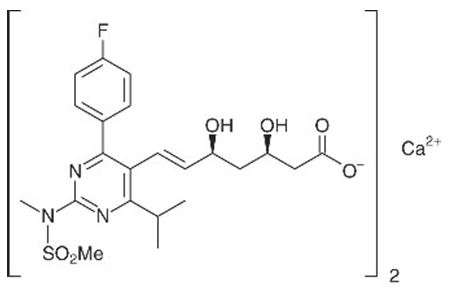
The empirical formula for rosuvastatin calcium is (C
CRESTOR tablets for oral use contain rosuvastatin 5 mg, 10 mg, 20 mg, or 40 mg (equivalent to 5.2 mg, 10.4 mg, 20.8 mg, and 41.6 mg rosuvastatin calcium) and the following inactive ingredients: crospovidone NF, hypromellose NF, lactose monohydrate NF, magnesium stearate NF, microcrystalline cellulose NF, red ferric oxide NF, titanium dioxide USP, triacetin NF, tribasic calcium phosphate NF and yellow ferric oxide.
7CLINICAL STUDIES
Primary Prevention of CV Disease
In the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) study, the effect of CRESTOR on the occurrence of major CV disease events was assessed in 17,802 males (≥50 years) and females (≥60 years) who had no clinically evident CV disease, LDL‑C levels <130 mg/dL and hsCRP levels ≥2 mg/L. The study population had an estimated baseline coronary heart disease risk of 11.6% over 10 years based on the Framingham risk criteria and included a high percentage of patients with additional risk factors such as hypertension (58%), low HDL‑C levels (23%), cigarette smoking (16%), or a family history of premature CHD (12%). Patients had a median baseline LDL‑C of 108 mg/dL and hsCRP of 4.3 mg/L. Patients were randomly assigned to placebo (n=8901) or CRESTOR 20 mg once daily (n=8901) and were followed for a mean duration of 2 years. The JUPITER study was stopped early by the Data Safety Monitoring Board due to meeting predefined stopping rules for efficacy in CRESTOR-treated subjects.
The primary end point was a composite end point consisting of the time-to-first occurrence of any of the following major CV events: CV death, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina or an arterial revascularization procedure.
CRESTOR significantly reduced the risk of major CV events (252 events in the placebo group vs. 142 events in the rosuvastatin group) with a statistically significant (p<0.001) relative risk reduction of 44% and absolute risk reduction of 1.2% (see Figure 1). The risk reduction for the primary end point was consistent across the following predefined subgroups: age, sex, race, smoking status, family history of premature CHD, body mass index, LDL‑C, HDL‑C, and hsCRP levels.
Figure 1. Time to First Occurrence of Major CV Events in JUPITER
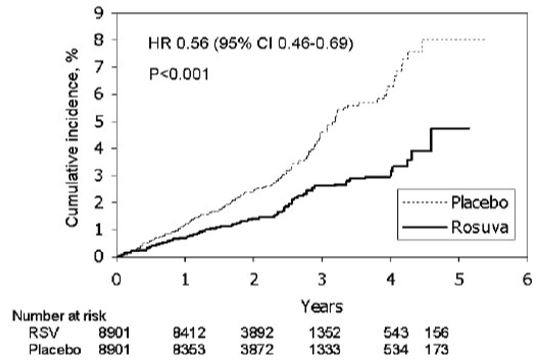
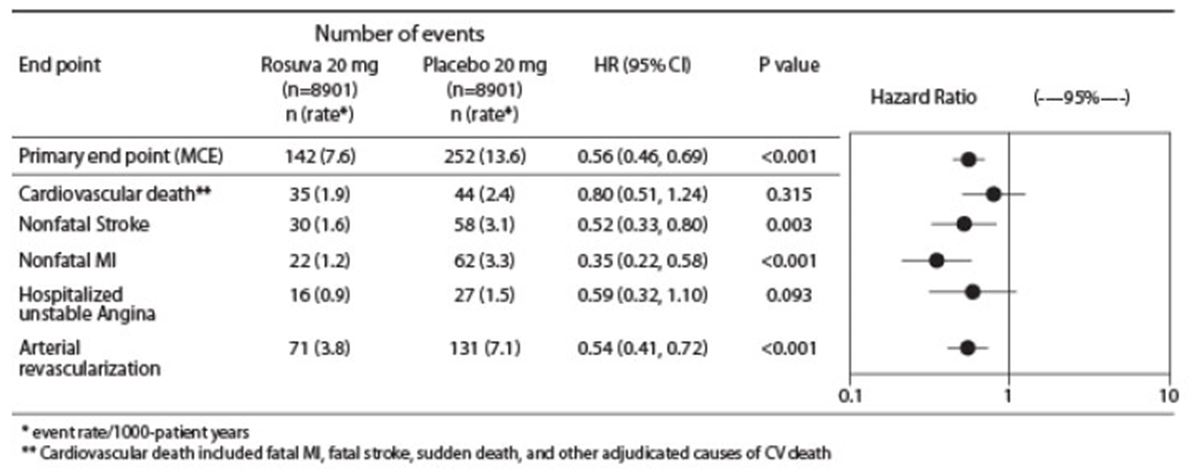
The individual components of the primary end point are presented in Figure 3. CRESTOR significantly reduced the risk of nonfatal myocardial infarction, nonfatal stroke, and arterial revascularization procedures. There were no significant treatment differences between the CRESTOR and placebo groups for death due to CV causes or hospitalizations for unstable angina.
CRESTOR significantly reduced the risk of myocardial infarction (6 fatal events and 62 nonfatal events in placebo-treated subjects vs. 9 fatal events and 22 nonfatal events in CRESTOR-treated subjects) and the risk of stroke (6 fatal events and 58 nonfatal events in placebo-treated subjects vs. 3 fatal events and 30 nonfatal events in CRESTOR-treated subjects).
In a post-hoc subgroup analysis of JUPITER subjects (rosuvastatin=725, placebo=680) with a hsCRP ≥2 mg/L and no other traditional risk factors (smoking, BP ≥140/90 or taking antihypertensives, low HDL‑C) other than age, after adjustment for high HDL‑C, there was no significant treatment benefit with CRESTOR treatment.
Figure 2. Major CV Events by Treatment Group in JUPITER
At one year, CRESTOR increased HDL‑C and reduced LDL‑C, hsCRP, total cholesterol and serum triglyceride levels (p<0.001 for all versus placebo).
Primary Hyperlipidemia in Adults
CRESTOR reduces Total‑C, LDL‑C, ApoB, non‑HDL‑C, and TG, and increases HDL‑C, in adult patients with hyperlipidemia and mixed dyslipidemia.
In a multicenter, double‑blind, placebo‑controlled study in patients with hyperlipidemia, CRESTOR given as a single daily dose (5 to 40 mg) for 6 weeks significantly reduced Total‑C, LDL‑C, non‑HDL‑C, and ApoB, across the dose range (Table 10).
CRESTOR was compared with the statins (atorvastatin, simvastatin, and pravastatin) in a multicenter, open-label, dose‑ranging study of 2,240 patients with hyperlipidemia or mixed dyslipidemia. After randomization, patients were treated for 6 weeks with a single daily dose of either CRESTOR, atorvastatin, simvastatin, or pravastatin (see Figure 3 and Table 11).
Figure 3. Percent LDL‑C Change by Dose of CRESTOR, Atorvastatin, Simvastatin, and Pravastatin at Week 6 in Adult Patients with Hyperlipidemia or Mixed Dyslipidemia
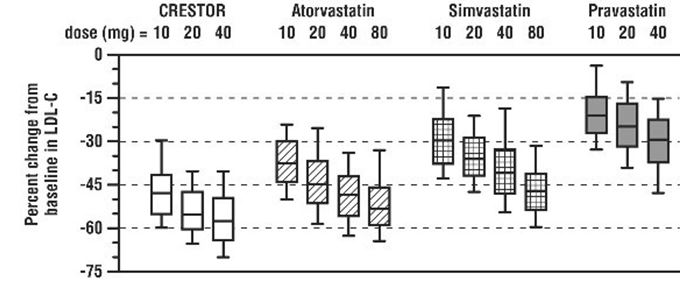
Box plots are a representation of the 25th, 50th, and 75th percentile values, with whiskers representing the 10th and 90th percentile values. Mean baseline LDL‑C: 189 mg/dL.
Slowing of the Progression of Atherosclerosis
In the
The annualized rate of change from baseline for the placebo group was +0.0131 mm/year (p<0.0001). The annualized rate of change from baseline for the group treated with CRESTOR was -0.0014 mm/year (p=0.32).
At an individual patient level in the group treated with CRESTOR, 52.1% of patients demonstrated an absence of disease progression (defined as a negative annualized rate of change), compared to 37.7% of patients in the placebo group.
HeFH in Adults
In a study of adult patients with HeFH (baseline mean LDL of 291 mg/dL), patients were randomized to CRESTOR 20 mg or atorvastatin 20 mg. The dose was increased at 6-week intervals. Significant LDL-C reductions from baseline were seen at each dose in both treatment groups (see Table 12).
HeFH in Pediatric Patients
In a double-blind, randomized, multicenter, placebo-controlled, 12-week study, 176 (97 male and 79 female) pediatric patients with HeFH were randomized to rosuvastatin 5 mg, 10 mg or 20 mg or placebo daily. Patients ranged in age from 10 to 17 years (median age of 14 years) with approximately 30% of the patients 10 to 13 years and approximately 17%, 18%, 40%, and 25% at Tanner stages II, III, IV, and V, respectively. Females were at least 1-year postmenarche. Mean LDL-C at baseline was 233 mg/dL (range of 129 to 399). The 12-week double-blind phase was followed by a 40-week open-label dose-titration phase, where all patients (n=173) received 5 mg, 10 mg or 20 mg rosuvastatin daily.
Rosuvastatin significantly reduced LDL-C (primary end point), total cholesterol and ApoB levels at each dose compared to placebo. Results are shown in Table 13 below.
Rosuvastatin was also studied in a two-year open‑label, uncontrolled, titration-to-goal trial that included 175 pediatric patients with HeFH who were 8 to 17 years old (79 males and 96 females). All patients had a documented genetic defect in the LDL receptor or in ApoB. Approximately 89% were White, 7% were Asian, 1% were Black or African American, and fewer than 1% were Hispanic or Latino ethnicity. Mean LDL-C at baseline was 236 mg/dL. Fifty-eight (33%) patients were prepubertal at baseline. The starting rosuvastatin dosage for all pediatric patients was 5 mg once daily. Pediatric patients aged 8 to less than 10 years (n=41 at baseline) could titrate to a maximum dosage of 10 mg once daily, and pediatric patients aged 10 to 17 years could titrate to a maximum dosage of 20 mg once daily.
The reductions in LDL‑C from baseline were generally consistent across age groups within the trial as well as with previous experience in both adult and pediatric controlled trials.
HoFH in Adult and Pediatric Patients
In an open-label, forced-titration study, HoFH patients (n=40, 8‑63 years) were evaluated for their response to CRESTOR 20 to 40 mg titrated at a 6‑week interval. In the overall population, the mean LDL‑C reduction from baseline was 22%. About one-third of the patients benefited from increasing their dose from 20 mg to 40 mg with further LDL‑C lowering of greater than 6%. In the 27 patients with at least a 15% reduction in LDL‑C, the mean LDL‑C reduction was 30% (median 28% reduction). Among 13 patients with an LDL‑C reduction of <15%, 3 had no change or an increase in LDL‑C. Reductions in LDL‑C of 15% or greater were observed in 3 of 5 patients with known receptor negative status.
HoFH in Pediatric Patients
CRESTOR was studied in a randomized, double-blind, placebo-controlled, multicenter, cross-over study in 14 pediatric patients with HoFH. The study included a 4‑week dietary lead‑in phase during which patients received CRESTOR 10 mg daily, a cross‑over phase that included two 6‑week treatment periods with either CRESTOR 20 mg or placebo in random order, followed by a 12‑week open‑label phase during which all patients received CRESTOR 20 mg. Patients ranged in age from 7 to 15 years of age (median 11 years), 50% were male, 71% were White, 21% were Asian, 7% were Black or African American, and no patients were of Hispanic or Latino ethnicity. Fifty percent were on apheresis therapy and 57% were taking ezetimibe. Patients who entered the study on apheresis therapy or ezetimibe continued the treatment throughout the entire study. Mean LDL-C at baseline was 416 mg/dL (range 152 to 716 mg/dL). A total of 13 patients completed both treatment periods of the randomized cross-over phase; one patient withdrew consent due to inability to have blood drawn during the cross-over phase.
CRESTOR 20 mg significantly reduced LDL-C, total cholesterol, ApoB, and non-HDL-C compared to placebo (see Table 14).
Primary Dysbetalipoproteinemia in Adults
In a randomized, multicenter, double-blind crossover-study, 32 adult patients (27 with є2/є2 and 4 with apo E mutation [Arg145Cys] with primary dysbetalipoproteinemia entered a 6‑week dietary lead-in period on the NCEP Therapeutic Lifestyle Change (TLC) diet. Following dietary lead-in, patients were randomized to a sequence of treatments for 6 weeks each: rosuvastatin 10 mg followed by rosuvastatin 20 mg or rosuvastatin 20 mg followed by rosuvastatin 10 mg. CRESTOR reduced non-HDL‑C (primary end point) and circulating remnant lipoprotein levels. Results are shown in the table below.
Hypertriglyceridemia in Adults
In a double-blind, placebo-controlled study in adult patients with baseline TG levels from 273 to 817 mg/dL, CRESTOR given as a single daily dose (5 to 40 mg) over 6 weeks significantly reduced serum TG levels (Table 16).
8HOW SUPPLIED/STORAGE AND HANDLING
CRESTOR tablets are supplied as:
Storage
Store at controlled room temperature, 20ºC to 25ºC (68ºF to 77ºF); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature]. Protect from moisture.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Myopathy and Rhabdomyolysis
Advise patients that CRESTOR may cause myopathy and rhabdomyolysis. Inform patients that the risk is also increased when taking certain types of medication and they should discuss all medication, both prescription and over-the-counter, with their healthcare provider. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever
Hepatic Dysfunction
Inform patients that CRESTOR may cause liver enzyme elevations and possibly liver failure. Advise patients to promptly report fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice
Increases in HbA1c and Fasting Serum Glucose Levels
Inform patients that increases in HbA1c and fasting serum glucose levels may occur with CRESTOR. Encourage patients to optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices
Pregnancy
Advise pregnant patients and patients who can become pregnant of the potential risk to a fetus. Advise patients to inform their healthcare provider of a known or suspected pregnancy to discuss if CRESTOR should be discontinued
Lactation
Advise patients that breastfeeding during treatment with CRESTOR is not recommended
Concomitant Use of Antacids
When taking CRESTOR with an aluminum and magnesium hydroxide combination antacid, administer CRESTOR at least 2 hours before the antacid
Missed Doses
If a dose is missed, advise patients not take an extra dose. Just resume the usual schedule
CRESTOR
© AstraZeneca XXXX
Licensed from SHIONOGI & CO., LTD., Osaka, Japan
Distributed by:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
10Package/Label Display Panel – 5 mg
NDC 0310-7560-90 90 tablets
CRESTOR®
rosuvastatin
5 mg tablets
Rx only
Mfd. for: AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
By: IPR Pharmaceuticals, Inc.
Canóvanas, PR 00729
Product of Switzerland
AstraZeneca

11Package/Label Display Panel – 10 mg
NDC 0310-7570-90 90 tablets
CRESTOR®
rosuvastatin
10 mg tablets
Rx only
Mfd. for: AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
By: IPR Pharmaceuticals, Inc.
Canóvanas, PR 00729
Product of Switzerland
AstraZeneca

12Package/Label Display Panel – 20 mg
NDC 0310-7580-90 90 tablets
CRESTOR®
rosuvastatin
20 mg tablets
Rx only
Mfd. for: AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
By: IPR Pharmaceuticals, Inc.
Canóvanas, PR 00729
Product of Switzerland
AstraZeneca

13Package/Label Display Panel – 40 mg
NDC 0310-7590-30 30 tablets
CRESTOR®
rosuvastatin
40 mg tablets
Rx only
Mfd. for: AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
By: IPR Pharmaceuticals Inc
Canóvanas, PR 00729
Product of Switzerland
AstraZeneca
