Bludigo
What is Bludigo (Indigotindisulfonate)?
Approved To Treat
Related Clinical Trials
Summary: The primary objective of this study is to collect and evaluate clinical evidence supporting the safety and performance of the Indigo™ Aspiration System in a patient population with lower extremity acute limb ischemia (LE ALI).
Summary: The objective of The Neighborhood \& Health Study is to use a quasi-experimental mixed-methods approach to assess the impact of living in an agrihood-an agriculturally integrated community. This study follows a longitudinal cohort of residents of a newly developed neighborhood (the Indigo Neighborhood) and a geographically and socio-demographically matched neighborhood (the Elyson Neighborhood), b...
Summary: The objective of this study is to evaluate real world long-term functional outcomes, safety and performance of the Indigo Aspiration System for the treatment of pulmonary embolism (PE).
Related Latest Advances
Brand Information
- Cardiovascular Reactions
- Hypersensitivity Reactions
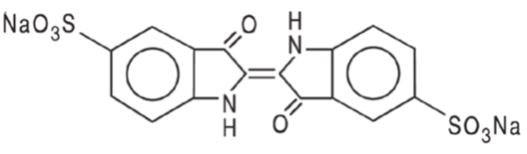
Table 3. Summary of Proportion of Responders by Ureter and Reviewer or Surgeon in Patients Receiving BLUDIGO 5 mL
BLUDIGO (indigotindisulfonate sodium injection, USP) 40 mg/5 mL (8 mg/mL) is a dark blue or bluish-purple solution supplied in a carton of 5 single-dose amber glass ampules (NDC 81284-315-05).
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
Store in original carton to protect from light.
Do not refrigerate or freeze.
Use immediately after opening ampule. Discard unused portion.
Advise the patient of the possibility of developing elevated blood pressure, hypotension, bradycardia, tachycardia, or atrioventricular block during or after the administration of BLUDIGO [see Warnings and Precautions (
Inform the patient that BLUDIGO may cause a blue discoloration of injection site and urine and that the discoloration should resolve within 48 hours. Advise the patient to inform the healthcare provider if the discoloration of the injection site is associated with other symptoms [see Adverse Reactions (
indigotindisulfonate
sodium Injection, USP
5 mL - Single dose ampule
indigotindisulfonate sodium
Injection, USP


