Izervay
What is Izervay (Avacincaptad Pegol)?
Approved To Treat
Related Clinical Trials
Summary: Age-related macular degeneration (AMD) is an eye disease which causes people to lose their vision over time. AMD damages the macula, which is in the middle of the retina - the light sensitive part at the back of the eye. In AMD, the cells in the macula die over time, usually over several years, leading to vision loss. When AMD gets worse, it can turn into either geographic atrophy (GA), neovascula...
Summary: This study is for people who have geographic atrophy due to age-related macular degeneration (AMD). AMD happens when the macula, the light-sensitive layer at the back of the eye called the retina, becomes damaged and causes a person's central vision to worsen. Geographic atrophy is an advanced form of AMD where cells in the retina waste away and die. Over time this can lead to permanent loss of vi...
Summary: The main purpose of this study is to assess the ocular and systemic safety and tolerability of RO7669330 in GA secondary to AMD after multiple unilateral intravitreal (IVT) doses.
Related Latest Advances
Brand Information
- Ocular and periocular infections
- Active intraocular inflammation
- Endophthalmitis and retinal detachments
- Neovascular AMD
- Increase in intraocular pressure
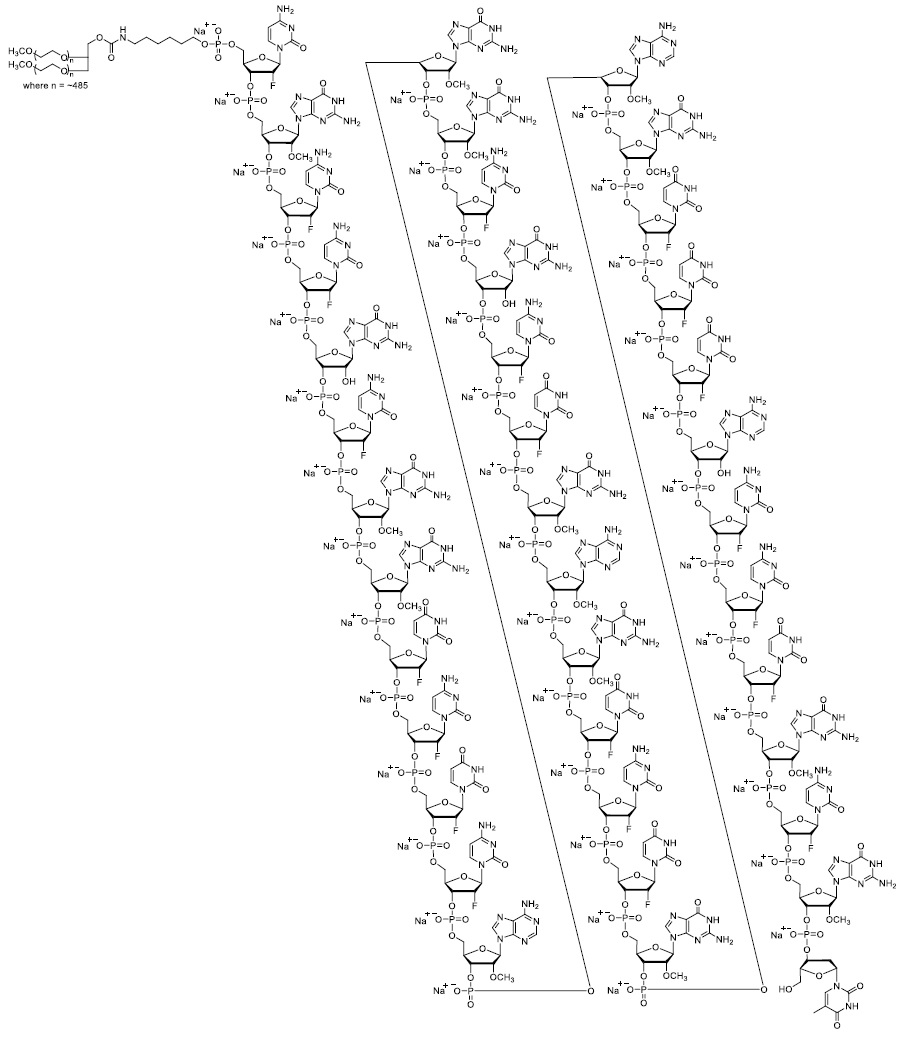
(avacincaptad pegol
intravitreal solution)
0.1 mL of 20 mg/mL
(avacincaptad pegol intravitreal solution)
(avacincaptad pegol
intravitreal solution)
0.1 mL of 20 mg/mL
(avacincaptad pegol intravitreal solution)