Scemblix
What is Scemblix (Asciminib)?
For many people living with chronic myeloid leukemia (CML), managing the disease means long-term treatment, frequent monitoring, and adapting to therapies that can lose effectiveness over time. Scemblix (asciminib) represents a newer and more targeted approach for patients whose cancer has not responded to earlier treatments. It brings hope to individuals who have struggled with resistance or intolerance to other medications, offering another chance at remission and stability in daily life.
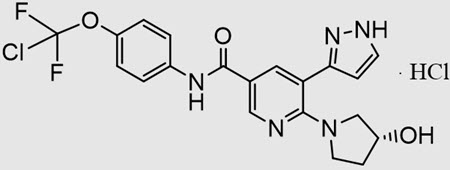
Scemblix is a tyrosine kinase inhibitor (TKI) specifically approved for adults with Philadelphia chromosome–positive chronic myeloid leukemia (Ph+ CML) in the chronic phase. Developed by Novartis, it belongs to a unique subclass known as a STAMP inhibitor, short for Specifically Targeting the ABL Myristoyl Pocket. Unlike traditional TKIs, Scemblix works in a novel way to control cancer growth, making it a valuable treatment for patients who have already received other TKIs such as imatinib, dasatinib, or nilotinib.
As one of the first drugs of its kind, Scemblix expands treatment options for people living with CML and reflects significant progress in personalized cancer therapy.
What does Scemblix do?
Scemblix is prescribed to treat adults with chronic myeloid leukemia (CML) in the chronic phase who have previously been treated with two or more other tyrosine kinase inhibitors. It is also approved for use in patients whose leukemia cells carry the T315I mutation, a genetic change that makes many other TKIs ineffective.
CML is a type of blood cancer that starts in the bone marrow and causes the body to produce too many white blood cells. This overproduction can crowd out healthy blood cells, leading to fatigue, infections, and other health problems.
By targeting the abnormal protein responsible for driving this uncontrolled growth, Scemblix helps reduce the number of leukemia cells and restore healthy blood cell balance. In clinical trials, many patients achieved major molecular responses meaning a significant reduction in cancer-related genetic material in their blood often with fewer side effects compared to older TKIs (FDA, 2021).
For patients who have exhausted other treatment options, Scemblix offers a renewed sense of control and hope for long-term disease management.
How does Scemblix work?
Unlike traditional CML medications that block the active site of the BCR-ABL1 protein, Scemblix works differently. It binds to a unique site on the BCR-ABL1 enzyme, called the myristoyl pocket, rather than the usual ATP-binding site targeted by older TKIs.
This distinctive mechanism allows Scemblix to shut down the activity of the abnormal protein more precisely. By doing so, it prevents leukemia cells from multiplying while sparing many normal cells.
The clinical significance of this is substantial: Scemblix can still work even when mutations like T315I make other TKIs ineffective. This means patients whose disease has become resistant to standard treatments have an alternative that directly attacks the resistant cancer pathway.
In simpler terms, Scemblix helps “turn off” the molecular switch that keeps leukemia cells growing uncontrollably offering a smarter and often better-tolerated way to control the disease.
Scemblix side effects
As with all cancer therapies, Scemblix can cause side effects, although not everyone experiences them. Many are manageable and lessen as the body adjusts to treatment.
Common side effects may include:
- Fatigue or weakness[Text Wrapping Break]
- Headache[Text Wrapping Break]
- Muscle or joint pain[Text Wrapping Break]
- Nausea or stomach discomfort[Text Wrapping Break]
- Rash or mild skin reactions[Text Wrapping Break]
- Upper respiratory infections
Serious side effects, though less common, can include:
- Low blood cell counts (anemia, neutropenia, or thrombocytopenia)[Text Wrapping Break]
- Elevated liver enzyme levels[Text Wrapping Break]
- High blood pressure[Text Wrapping Break]
- Pancreatitis (inflammation of the pancreas)[Text Wrapping Break]
- Allergic reactions such as swelling, rash, or difficulty breathing
Contact a doctor if you experience severe fatigue, fever, unusual bleeding, or shortness of breath. Inform your doctor about all medications and supplements to prevent harmful interactions.
Scemblix is not for those with severe liver issues, or who are pregnant or breastfeeding. Women of childbearing potential need contraception during and after treatment.
Scemblix dosage
Scemblix is an oral tablet taken once or twice daily, with or without food, swallowed whole. Dosing is determined by the doctor based on prior treatments, response, and overall health.
Regular medical monitoring is crucial. Physicians conduct blood tests (for cell counts, platelets), liver function tests (for medication safety), and molecular tests (for BCR-ABL1 levels and treatment effectiveness).
Older adults or those with liver disease may require closer monitoring. Side effects may necessitate dosage adjustments or treatment pauses.
Does Scemblix have a generic version?
As of 2025, Scemblix (asciminib) does not have a generic version available. It is currently a patented brand-name medication manufactured by Novartis and approved by the U.S. Food and Drug Administration (FDA) in 2021. However, international versions may exist in other markets.
Scemblix, a new targeted therapy, won’t have generics until patent expiration. Once available, generics will offer comparable safety and efficacy. Patients can explore financial assistance or insurance for cost management.
Conclusion
Scemblix (asciminib) represents a major step forward in the treatment of chronic myeloid leukemia, especially for patients who have struggled with resistance to older medications. By targeting a new molecular site, it provides a fresh way to control CML with precision and potentially fewer side effects.
While treatment with Scemblix requires ongoing monitoring and commitment, it offers hope for long-term disease control and improved quality of life. Every patient’s journey is different, but with guidance from a skilled healthcare provider and consistent follow-up care, Scemblix can be a powerful ally in managing CML and helping patients move forward with greater confidence and stability.
References
- U.S. Food and Drug Administration (FDA). (2021). FDA approves asciminib for Philadelphia chromosome-positive chronic myeloid leukemia. Retrieved from https://www.fda.gov[Text Wrapping Break]
- Mayo Clinic. (2024). Asciminib (oral route): Drug information and side effects. Retrieved from https://www.mayoclinic.org[Text Wrapping Break]
- MedlinePlus. (2024). Asciminib – Medication guide. National Library of Medicine. Retrieved from https://medlineplus.gov[Text Wrapping Break]
- National Cancer Institute (NCI). (2024). Chronic myeloid leukemia treatment (PDQ®)–Patient version. Retrieved from https://www.cancer.gov[Text Wrapping Break]
Approved To Treat
Related Clinical Trials
Summary: The purpose of the study is to see if a study drug called asciminib is safe and okay for people to take after they've had treatment for a type of blood cancer called Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia (Ph+ B-ALL). We're looking at two groups of adults: one group had an Allogeneic Stem Cell Transplant (alloHCT) cohort A, and the other group had chimeric antigen receptor T...
Summary: This research study is evaluating a drug called ABL001 taken in combination with dasatinib (Sprycel®) and prednisone (a steroid) as a possible treatment for B-cell Acute Lymphoblastic Leukemia that is BCR-ABL positive (BCR-ABL+ B-ALL) or Chronic Myeloid Leukemia (CML) in lymphoid blast crisis. BCR-ABL+ B-ALL is also called Philadelphia chromosome positive Acute Lymphoblastic Leukemia (Ph+ ALL). It...
Summary: This is a long term safety study for patients who have completed a Novartis sponsored asciminib study and are judged by the investigator to benefit from continued treatment
Related Latest Advances
Brand Information
- Newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML) in chronic phase (CP).
- Previously treated Ph+ CML in CP.
- Ph+ CML in CP with the T315I mutation.
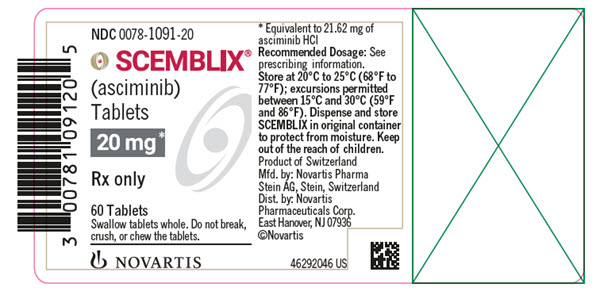
- 20 mg asciminib film-coated tablets: pale yellow, unscored, round, biconvex, with beveled edges, film-coated tablet debossed with “20” on one side and the “Novartis” logo on the other side.
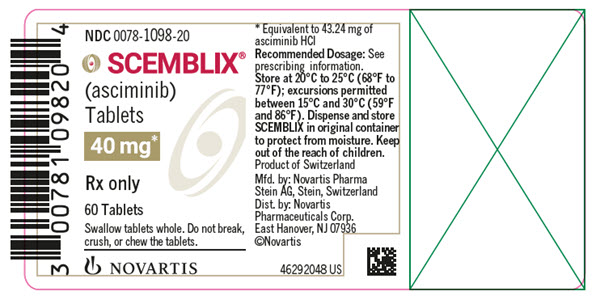
- 40 mg asciminib film-coated tablets: violet white, unscored, round, biconvex, with beveled edges, film-coated tablet debossed with “40” on one side and the “Novartis” logo on the other side.
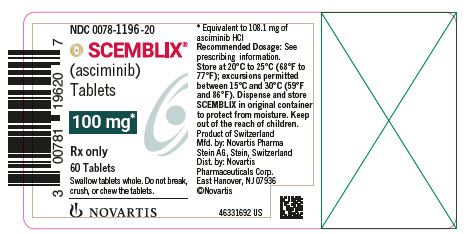
- 100 mg asciminib film-coated tablets: light red, unscored, round, biconvex, with beveled edges, film-coated tablet debossed with “100” on one side and the “Novartis” logo on the other side.
- Myelosuppression
- Pancreatic Toxicity
- Hypertension
- Hypersensitivity
- Cardiovascular Toxicity
- SCEMBLIX (asciminib) 20 mg film-coated tablets are supplied as pale yellow, unscored, round, biconvex, with beveled edges, film-coated tablets containing 20 mg of asciminib. Each tablet is debossed with “20” on one side and the “Novartis” logo on the other side.
- SCEMBLIX (asciminib) 40 mg film-coated tablets are supplied as violet white, unscored, round, biconvex, with beveled edges, film-coated tablets containing 40 mg of asciminib. Each tablet is debossed with “40” on one side and the “Novartis” logo on the other side.
- SCEMBLIX (asciminib) 100 mg film-coated tablets are supplied as light red, unscored, round, biconvex, with beveled edges, film-coated tablets containing 100 mg of asciminib. Each tablet is debossed with “100” on one side and the “Novartis” logo on the other side.