Brand Name
Vivjoa
Generic Name
Oteseconazole
View Brand Information FDA approval date: July 11, 2022
Classification: Azole Antifungal
Form: Capsule
What is Vivjoa (Oteseconazole)?
VIVJOA™ is an azole antifungal indicated to reduce the incidence of recurrent vulvovaginal candidiasis in females with a history of RVVC who are NOT of reproductive potential.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Platform Trial For Cryptococcal Meningitis
Summary: Cryptococcal meningitis is a fungal infection that causes a severe syndrome of meningitis that is 100% fatal without antifungal therapy. Even with antifungal therapy, mortality rates remain high, especially in low and middle income countries where the ongoing HIV/AIDS pandemic increases the risk of cryptococcosis among persons living with HIV infection. The combination of amphotericin and flucytos...
Related Latest Advances
Brand Information
VIVJOA (oteseconazole)
1DOSAGE FORMS AND STRENGTHS
VIVJOA Capsules: 150 mg of oteseconazole in lavender hard gelatin capsules imprinted with OTE 150 in black ink.
Fluconazole is not supplied in the carton.
2CONTRAINDICATIONS
VIVJOA is contraindicated in:
- Females of reproductive potential
- Pregnant and lactating women
- Patients with known hypersensitivity to oteseconazole.
3DESCRIPTION
VIVJOA (oteseconazole capsules) contains oteseconazole which is an oral azole antifungal agent.
The chemical name of oteseconazole is (
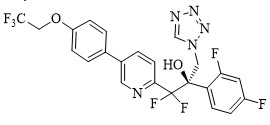
Oteseconazole is a white to off-white crystalline powder and is practically insoluble in water within a pH range of 1 to 9 but is soluble in a variety of organic solvents.
Each oteseconazole capsule, for oral use, contains 150 mg oteseconazole and the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, lactose, magnesium stearate, silicified microcrystalline cellulose, and sodium lauryl sulfate. Capsule shell and print constituents: FD&C Blue #1, FD&C Red #3, gelatin, Opacode SW-9008/SW-9009 and titanium dioxide. Contains no ingredient made from a gluten-containing grain (wheat, barley, or rye).
4PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
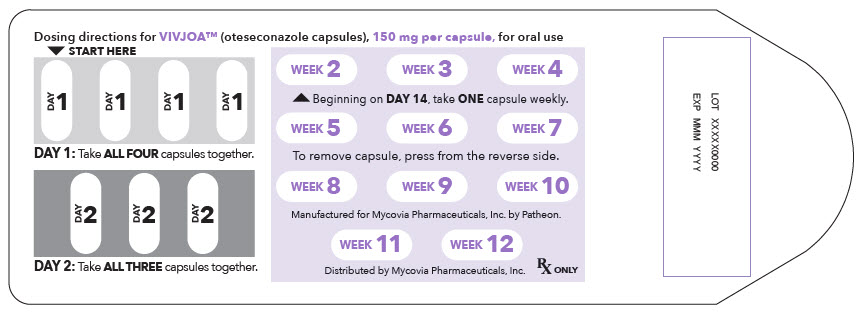
5PRINCIPAL DISPLAY PANEL - 150 mg Capsule Blister Pack Label
Dosing directions for VIVJOA™ (oteseconazole capsules), 150 mg per capsule, for oral use
▼ START HERE
DAY
DAY 1: Take ALL FOUR capsules together.
DAY
DAY 2: Take ALL THREE capsules together.
WEEK
Beginning on DAY 14, take ONE capsule weekly.
WEEK
To remove capsule, press from the reverse side.
WEEK
Manufactured for Mycovia Pharmaceuticals, Inc. by Patheon.
WEEK
Distributed by Mycovia Pharmaceuticals, Inc.
Rx ONLY
LOT XXXXX0000
6PRINCIPAL DISPLAY PANEL - 150 mg Capsule Blister Pack Container
NDC 74695-823-18
18 CAPSULES
Press and
vivjoa™
See package insert for full Prescribing Information.
STEP 1 Press and hold button.
STEP 2 Pull out medication card.
Pull out here.

7PRINCIPAL DISPLAY PANEL - 150 mg Capsule Blister Pack Container Carton
NDC 74695-823-18
See package insert for full Prescribing Information.
vivjoa™
150 mg per capsule, for oral use
18 CAPSULES

