Generic Name
MedroxyPROGESTERone Acetate
Brand Names
Prempro, MedroxyPROGESTERone, Depo-Provera, Provera, Depo-SubQ Provera, Premphase
FDA approval date: June 03, 1959
Classification: Progestin
Form: Injection, Tablet, Kit
What is Prempro (MedroxyPROGESTERone Acetate)?
Medroxyprogesterone Acetate Tablets USP are indicated for the treatment of secondary amenorrhea and abnormal uterine bleeding due to hormonal imbalance in the absence of organic pathology, such as fibroids or uterine cancer. They are also indicated for use in the prevention of endometrial hyperplasia in nonhysterectomized postmenopausal women who are receiving daily oral conjugated estrogens.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Premphase (conjugated estrogens and medroxyprogesterone acetate)
WARNING: CARDIOVASCULAR DISORDERS, BREAST CANCER, ENDOMETRIAL CANCER and PROBABLE DEMENTIA
Estrogen Plus Progestin Therapy
Cardiovascular Disorders and Probable Dementia
Estrogen plus progestin therapy should not be used for the prevention of cardiovascular disease or dementia
The Women's Health Initiative (WHI) estrogen plus progestin substudy reported an increased risk of deep vein thrombosis (DVT), pulmonary embolism (PE), stroke and myocardial infarction (MI) in postmenopausal women (50 to 79 years of age) during 5.6 years of treatment with daily oral conjugated estrogen (CE) (0.625 mg) combined with medroxyprogesterone acetate (MPA) (2.5 mg), relative to placebo
The WHI Memory Study (WHIMS) estrogen plus progestin ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 4 years of treatment with daily CE (0.625 mg) combined with MPA (2.5 mg), relative to placebo. It is unknown whether this finding applies to younger postmenopausal women
Breast Cancer
The WHI estrogen plus progestin substudy also demonstrated an increased risk of invasive breast cancer
In the absence of comparable data, these risks should be assumed to be similar for other doses of CE and MPA and other combinations and dosage forms of estrogens and progestins.
Estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
Estrogen-Alone Therapy
Endometrial Cancer
There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding
Cardiovascular Disorders and Probable Dementia
Estrogen-alone therapy should not be used for the prevention of cardiovascular disease or dementia
The WHI estrogen-alone substudy reported increased risks of stroke and DVT in postmenopausal women (50 to 79 years of age) during 7.1 years of treatment with daily oral CE (0.625 mg)-alone, relative to placebo
The WHIMS estrogen-alone ancillary study of WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 5.2 years of treatment with daily CE (0.625 mg)-alone, relative to placebo. It is unknown whether this finding applies to younger postmenopausal women
In the absence of comparable data, these risks should be assumed to be similar for other doses of CE and other dosage forms of estrogens.
Estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
1DOSAGE AND ADMINISTRATION
Use of estrogen-alone, or in combination with a progestin, should be with the lowest effective dose and for the shortest duration consistent with treatment goals and risks for the individual woman. Postmenopausal women should be re-evaluated periodically as clinically appropriate to determine if treatment is still necessary.
1.1Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause
PREMPRO therapy consists of a single tablet to be taken orally once daily.
PREMPHASE therapy consists of two separate tablets: one maroon 0.625 mg Premarin [conjugated estrogens (CE)] tablet taken daily on days 1 through 14 and one light-blue tablet containing 0.625 mg CE and 5 mg of medroxyprogesterone acetate (MPA) taken on days 15 through 28.
1.2Treatment of Moderate to Severe Vulvar and Vaginal Atrophy due to Menopause
PREMPRO therapy consists of a single tablet to be taken orally once daily.
PREMPHASE therapy consists of two separate tablets: one maroon 0.625 mg CE tablet taken daily on days 1 through 14 and one light-blue tablet containing 0.625 mg CE and 5 mg MPA taken on days 15 through 28.
When prescribing solely for the treatment of moderate to severe vulvar and vaginal atrophy, topical vaginal products should be considered.
1.3Prevention of Postmenopausal Osteoporosis
PREMPRO therapy consists of a single tablet to be taken orally once daily.
PREMPHASE therapy consists of two separate tablets: one maroon 0.625 mg CE tablet taken daily on days 1 through 14 and one light-blue tablet containing 0.625 mg CE and 5 mg of MPA taken on days 15 through 28.
When prescribing solely for the prevention of postmenopausal osteoporosis, therapy should only be considered for women at significant risk of osteoporosis and non-estrogen medications should be carefully considered.
2CONTRAINDICATIONS
PREMPRO and PREMPHASE are contraindicated in women with any of the following conditions:
- Undiagnosed abnormal genital bleeding
- Breast cancer or a history of breast cancer
- Estrogen-dependent neoplasia
- Active DVT, PE, or a history of these conditions
- Active arterial thromboembolic disease (for example, stroke and MI), or a history of these conditions
- Known anaphylactic reaction or angioedema with PREMPRO/PREMPHASE
- Hepatic impairment or disease
- Protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders
3ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Cardiovascular Disorders
- Malignant Neoplasms
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trial of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In a 1-year clinical trial that included 678 postmenopausal women treated with PREMPRO and 351 postmenopausal women treated with PREMPHASE, the following adverse reactions occurred at a rate ≥ 1%, see
In addition, phargyngitis and sinusitis were reported as two of the more frequent adverse events (>5%) in the PREMPRO clinical study. For pharyngitis, of the 121 events, six events were considered by the investigator causally related to study drug. For sinusitis, of the 73 events, one event was considered as casually related to study drug.
During the first year of a 2-year clinical trial with postmenopausal women between 40 and 65 years of age (88% Caucasian), 989 postmenopausal women received continuous regimens of PREMPRO, and 332 received placebo tablets. Table 2 summarizes adverse reactions that occurred at a rate ≥ 1% in at least 1 treatment group.
In addition, the following events were considered as related to the study drug with an incidence less than 1%, including accidental injury, infection, myalgia, cough increased, rhinitis, sinusitis, and upper respiratory infection.
3.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of PREMPRO or PREMPHASE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
4DRUG INTERACTIONS
Data from a single-dose drug-drug interaction study involving CE and MPA indicate that the pharmacokinetic disposition of both drugs is not altered when the drugs are coadministered. No other clinical drug-drug interaction studies have been conducted with CE plus MPA.
4.1Metabolic Interactions
In vitro and in vivo studies have shown that estrogens are metabolized partially by cytochrome P450 3A4 (CYP3A4). Therefore, inducers or inhibitors of CYP3A4 may affect estrogen drug metabolism. Inducers of CYP3A4, such as St. John's wort (Hypericum perforatum) preparations, phenobarbital, carbamazepine, and rifampin, may reduce plasma concentrations of estrogens, possibly resulting in a decrease in therapeutic effects and/or changes in the uterine bleeding profile. Inhibitors of CYP3A4, such as erythromycin, clarithromycin, ketoconazole, itraconazole, ritonavir and grapefruit juice, may increase plasma concentrations of estrogens and may result in side effects.
Aminoglutethimide administered concomitantly with MPA may significantly depress the bioavailability of MPA.
5OVERDOSAGE
Overdosage of estrogen plus progestin may cause nausea, vomiting, breast tenderness, abdominal pain, drowsiness and fatigue, and withdrawal bleeding may occur in women. Treatment of overdose consists of discontinuation of PREMPRO or PREMPHASE therapy with institution of appropriate symptomatic care.
6DESCRIPTION
Premarin (conjugated estrogens tablets, USP) for oral administration contains a mixture obtained exclusively from natural sources, occurring as the sodium salts of water-soluble estrogen sulfates blended to represent the average composition of material derived from pregnant mares' urine. It is a mixture of sodium estrone sulfate and sodium equilin sulfate. It contains as concomitant components, as sodium sulfate conjugates, 17 α-dihydroequilin, 17 α-estradiol and 17 β-dihydroequilin.
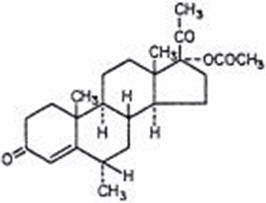
Medroxyprogesterone acetate is a derivative of progesterone. It is a white to off-white, odorless, crystalline powder, stable in air, melting between 200°C and 210°C. It is freely soluble in chloroform, soluble in acetone and in dioxane, sparingly soluble in alcohol and in methanol, slightly soluble in ether, and insoluble in water. The chemical name for MPA is pregn-4-ene-3, 20-dione, 17-(acetyloxy)-6-methyl-, (6α)-. Its molecular formula is C
PREMPRO 0.3 mg/1.5 mg and 0.45 mg/1.5 mg tablets contain the following inactive ingredients: calcium phosphate tribasic, microcrystalline cellulose, carnauba wax, hypromellose, hydroxypropyl cellulose, sucrose, Eudragit NE 30D, lactose monohydrate, magnesium stearate, polyethylene glycol, titanium dioxide, yellow iron oxide, propylene glycol and black iron oxide.
PREMPRO 0.625 mg/2.5 mg tablets contain the following inactive ingredients: calcium phosphate tribasic, microcrystalline cellulose, carnauba wax, hypromellose, hydroxypropyl cellulose, sucrose, Eudragit NE 30D, lactose monohydrate, magnesium stearate, polyethylene glycol, propylene glycol, titanium dioxide, red iron oxide, yellow iron oxide, and black iron oxide.
PREMPRO 0.625 mg/5 mg tablets contain the following inactive ingredients: calcium phosphate tribasic, carnauba wax, Eudragit NE 30D, hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, sucrose, titanium dioxide, triethyl citrate, FD&C Blue No. 2, black iron oxide, and propylene glycol.
7REFERENCES
- Rossouw JE, et al. Postmenopausal Hormone Therapy and Risk of Cardiovascular Disease by Age and Years Since Menopause.
- Hsia J, et al. Conjugated Equine Estrogens and Coronary Heart Disease.
- Cushman M, et al. Estrogen Plus Progestin and Risk of Venous Thrombosis.
- Curb JD, et al. Venous Thrombosis and Conjugated Equine Estrogen in Women Without a Uterus.
- Stefanick ML, et al. Effects of Conjugated Equine Estrogens on Breast Cancer and Mammography Screening in Postmenopausal Women With Hysterectomy.
- Chlebowski RT, et al. Influence of Estrogen Plus Progestin on Breast Cancer and Mammography in Healthy Postmenopausal Women.
- Anderson GL, et al. Effects of Estrogen Plus Progestin on Gynecologic Cancers and Associated Diagnostic Procedures.
- Shumaker SA, et al. Conjugated Equine Estrogens and Incidence of Probable Dementia and Mild Cognitive Impairment in Postmenopausal Women.
- Jackson RD, et al. Effects of Conjugated Equine Estrogen on Risk of Fractures and BMD in Postmenopausal Women With Hysterectomy: Results From the Women's Health Initiative Randomized Trial.
- Hendrix SL, et al. Effects of Conjugated Equine Estrogen on Stroke in the Women's Health Initiative.
8PATIENT COUNSELING INFORMATION
Advise the patients to read the
8.1Abnormal Vaginal Bleeding
Inform postmenopausal women of the importance of reporting abnormal vaginal bleeding to their healthcare provider as soon as possible
8.2Possible Serious Adverse Reactions with Estrogen Plus Progestin Therapy
Inform postmenopausal women of possible serious adverse reactions of estrogen plus progestin therapy including Cardiovascular Disorders, Malignant Neoplasms, and Probable Dementia
8.3Possible Less Serious but Common Adverse Reactions with Estrogen Plus Progestin Therapy
Inform postmenopausal women of possible less serious but common adverse reactions of estrogen plus progestin therapy such as headache, breast pain and tenderness, nausea and vomiting.
9PATIENT INFORMATION
PREMPRO
Read this PATIENT INFORMATION before you start taking PREMPRO or PREMPHASE and read what you get each time you refill your PREMPRO or PREMPHASE prescription. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is PREMPRO or PREMPHASE?
PREMPRO or PREMPHASE are medicines that contain two kinds of hormones, estrogens and a progestin.
What is PREMPRO or PREMPHASE used for?
PREMPRO or PREMPHASE is used after menopause to:
- Reduce moderate to severe hot flushes
Estrogens are hormones made by a woman's ovaries. The ovaries normally stop making estrogens when a woman is between 45 and 55 years old. This drop in body estrogen levels causes the "change of life" or menopause (the end of monthly menstrual periods). Sometimes, both ovaries are removed during an operation before natural menopause takes place. The sudden drop in estrogen levels causes "surgical menopause."
When the estrogen levels begin dropping, some women get very uncomfortable symptoms, such as feelings of warmth in the face, neck, and chest, or sudden strong feelings of heat and sweating ("hot flushes"). In some women the symptoms are mild, and they will not need to take estrogens. In other women, symptoms can be more severe. - Treat menopausal changes in and around the vagina
You and your healthcare provider should talk regularly about whether you still need treatment with PREMPRO or PREMPHASE to control these problems. If you use PREMPRO or PREMPHASE only to treat your menopausal changes in and around your vagina, talk with your healthcare provider about whether a topical vaginal product would be better for you. - Help reduce your chances of getting osteoporosis (thin weak bones)
Osteoporosis from menopause is a thinning of the bones that makes them weaker and easier to break. If you use PREMPRO or PREMPHASE only to prevent osteoporosis due to menopause, talk with your healthcare provider about whether a different treatment or medicine without estrogens might be better for you. Weight-bearing exercise, like walking or running, and taking calcium (1500 mg per day of elemental calcium) and vitamin D (400–800 IU per day) supplements may also lower your chances of getting postmenopausal osteoporosis. It is important to talk about exercise and supplements with your healthcare provider before starting them.
You and your healthcare provider should talk regularly about whether you still need treatment with PREMPRO or PREMPHASE.
Who should not take PREMPRO or PREMPHASE?
Do not take PREMPRO or PREMPHASE if you have had your uterus (womb) removed (hysterectomy).
PREMPRO and PREMPHASE contain a progestin to decrease the chance of getting cancer of the uterus. If you do not have a uterus, you do not need a progestin and you should not take PREMPRO or PREMPHASE.
Do not take PREMPRO or PREMPHASE if you:
- Have unusual vaginal bleeding
- Currently have or have had certain cancers
Estrogens may increase the chance of getting certain types of cancers, including cancer of the breast or uterus. If you have or have had cancer, talk with your healthcare provider about whether you should use PREMPRO or PREMPHASE. - Had a stroke or heart attack
- Currently have or have had blood clots
- Currently have or have had liver problems
- Have been diagnosed with a bleeding disorder
- Are allergic to PREMPRO or PREMPHASE or any of their ingredients
See the list of ingredients in PREMPRO and PREMPHASE at the end of this leaflet.
Tell your healthcare provider
- If you have any unusual vaginal bleeding
Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause. - About all of your medical problems
Your healthcare provider may need to check you more carefully if you have certain conditions, such as asthma (wheezing), epilepsy (seizures), diabetes, migraine, endometriosis, lupus, problems with your heart, liver, thyroid, kidneys, or have high calcium levels in your blood. - About all the medicines you take
This includes prescription and nonprescription medicines, vitamins, and herbal supplements. Some medicines may affect how PREMPRO or PREMPHASE works. PREMPRO or PREMPHASE may also affect how your other medicines work. - If you are going to have surgery or will be on bedrest
You may need to stop taking PREMPRO or PREMPHASE. - If you are pregnant or think you may be pregnant
PREMPRO and PREMPHASE are not for pregnant women. - If you are breastfeeding
The hormones in PREMPRO and PREMPHASE can pass into your breast milk.
How should I take PREMPRO or PREMPHASE?
- Take one PREMPRO or PREMPHASE tablet at the same time each day
- If you miss a dose, take it as soon as possible
- Estrogens should be used at the lowest dose possible for your treatment only as long as needed
What are the possible side effects of PREMPRO or PREMPHASE?
Side effects are grouped by how serious they are and how often they happen when you are treated.
Serious, but less common side effects include:
- Heart attack
- Stroke
- Blood clots
- Breast cancer
- Cancer of the lining of the uterus (womb)
- Cancer of the ovary
- Dementia
- High or low blood calcium
- Gallbladder disease
- Visual abnormalities
- High blood pressure
- High levels of fat (triglycerides) in your blood
- Liver problems
- Changes in your thyroid hormone levels
- Fluid retention
- Cancer changes of endometriosis
- Enlargement of benign tumors of the uterus ("fibroids")
- Severe allergic reactions
- Changes in certain laboratory test results, such as high blood sugar
Call your healthcare provider right away if you get any of the following warning signs or any other unusual symptoms that concern you:
- New breast lumps
- Unusual vaginal bleeding
- Changes in vision or speech
- Sudden new severe headaches
- Severe pains in your chest or legs with or without shortness of breath, weakness and fatigue
- Swelling of the face, lips, and tongue with or without red itchy bumps
Common side effects of PREMPRO or PREMPHASE include:
- Headache
- Breast pain
- Irregular vaginal bleeding or spotting
- Stomach or abdominal cramps, bloating
- Nausea and vomiting
- Hair loss
- Fluid retention
- Vaginal yeast infection
These are not all the possible side effects of PREMPRO or PREMPHASE. For more information, ask your healthcare provider or pharmacist for advice about side effects. You may report side effects to Pfizer Inc. at 1-800-438-1985 or to FDA at 1-800-FDA-1088.
What can I do to lower my chances of getting a serious side effect with PREMPRO or PREMPHASE?
- Talk with your healthcare provider regularly about whether you should continue taking PREMPRO or PREMPHASE
- See your healthcare provider right away if you get vaginal bleeding while taking PREMPRO or PREMPHASE
- Have a pelvic exam, breast exam and mammogram (breast X-ray) every year unless your healthcare provider tells you something else
- If you have high blood pressure, high cholesterol (fat in the blood), diabetes, are overweight, or if you use tobacco, you may have higher chances for getting heart disease
General Information about the safe and effective use of PREMPRO and PREMPHASE
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not take PREMPRO or PREMPHASE for conditions for which it was not prescribed. Do not give PREMPRO or PREMPHASE to other people, even if they have the same symptoms you have. It may harm them.
Keep PREMPRO and PREMPHASE out of the reach of children.
This leaflet provides a summary of the most important information about PREMPRO and PREMPHASE. If you would like more information, talk with your healthcare provider or pharmacist. You can ask for information about PREMPRO and PREMPHASE that is written for health professionals.
What are the ingredients in PREMPRO and PREMPHASE?
PREMPRO contains the same conjugated estrogens found in Premarin, which are a mixture of sodium estrone sulfate and sodium equilin sulfate and other components, including sodium sulfate conjugates, 17α-dihydroequilin, 17α-estradiol and 17β-dihydroequilin. PREMPRO also contains either 1.5, 2.5, or 5 mg of medroxyprogesterone acetate.
PREMPRO 0.3 mg/1.5 mg and 0.45 mg/1.5 mg tablets also contain calcium phosphate tribasic, microcrystalline cellulose, lactose monohydrate, carnauba wax, hypromellose, magnesium stearate, polyethylene glycol, sucrose, hydroxypropyl cellulose, Eudragit NE 30D, titanium dioxide, yellow iron oxide, propylene glycol and black iron oxide.
PREMPRO 0.625 mg/2.5 mg tablets also contain calcium phosphate tribasic, microcrystalline cellulose, carnauba wax, lactose monohydrate, hypromellose, magnesium stearate, polyethylene glycol, sucrose, hydroxypropyl cellulose, Eudragit NE 30D, propylene glycol, titanium dioxide, red iron oxide, yellow iron oxide, and black iron oxide.
PREMPRO 0.625 mg/5 mg tablets also contain calcium phosphate tribasic, carnauba wax, Eudragit NE 30D, hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, sucrose, titanium dioxide, triethyl citrate, FD&C Blue No. 2, black iron oxide, and propylene glycol.
PREMPHASE is two separate tablets. One tablet (maroon color) is 0.625 mg of Premarin, which is a mixture of sodium estrone sulfate and sodium equilin sulfate and other components, including sodium sulfate conjugates, 17 α-dihydroequilin, 17 α-estradiol and 17 β-dihydroequilin. The maroon tablet also contains calcium phosphate tribasic, carnauba wax, hydroxypropyl cellulose, microcrystalline cellulose, powdered cellulose, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, sucrose, titanium dioxide, propylene glycol, FD&C Blue No. 2, FD&C Red No. 40. The second tablet (light-blue color) contains 0.625 mg of the same ingredients as the maroon color tablet plus 5 mg of medroxyprogesterone acetate. The light-blue tablet also contains calcium phosphate tribasic, carnauba wax, Eudragit NE 30D, hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, sucrose, titanium dioxide, triethyl citrate, FD&C Blue No. 2, black iron oxide, and propylene glycol.
PREMPRO therapy consists of a single tablet to be taken once daily.
PREMPRO 0.3 mg/1.5 mg
Blister Card - Each carton includes 1 blister card containing 28 oval, cream tablets. Each tablet contains 0.3 mg of the conjugated estrogens found in Premarin tablets and 1.5 mg of medroxyprogesterone acetate for oral administration.
PREMPRO 0.45 mg/1.5 mg
Blister Card - Each carton includes 1 blister card containing 28 oval, gold tablets. Each tablet contains 0.45 mg of the conjugated estrogens found in Premarin tablets and 1.5 mg of medroxyprogesterone acetate for oral administration.
PREMPRO 0.625 mg/2.5 mg
Blister Card - Each carton includes 1 blister card containing 28 oval, peach tablets. Each tablet contains 0.625 mg of the conjugated estrogens found in Premarin tablets and 2.5 mg of medroxyprogesterone acetate for oral administration.
PREMPRO 0.625 mg/5 mg
Blister Card - Each carton includes 1 blister card containing 28 oval, light-blue tablets. Each tablet contains 0.625 mg of the conjugated estrogens found in Premarin tablets and 5 mg of medroxyprogesterone acetate for oral administration.
PREMPHASE therapy consists of two separate tablets; one maroon Premarin tablet taken daily on days 1 through 14 and one light-blue tablet taken on days 15 through 28.
Each carton includes 1 blister pack containing 28 tablets. One blister pack contains 14 oval, maroon Premarin tablets containing 0.625 mg of conjugated estrogens and 14 oval, light-blue tablets that contain 0.625 mg of the conjugated estrogens found in Premarin tablets and 5 mg of medroxyprogesterone acetate for oral administration.
The appearance of PREMPRO tablets is a trademark of Pfizer Inc.
The appearance of PREMARIN tablets is a trademark of Pfizer Inc. The appearance of the conjugated estrogens/medroxyprogesterone acetate combination tablets is a trademark.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].

LAB-0504-11.0
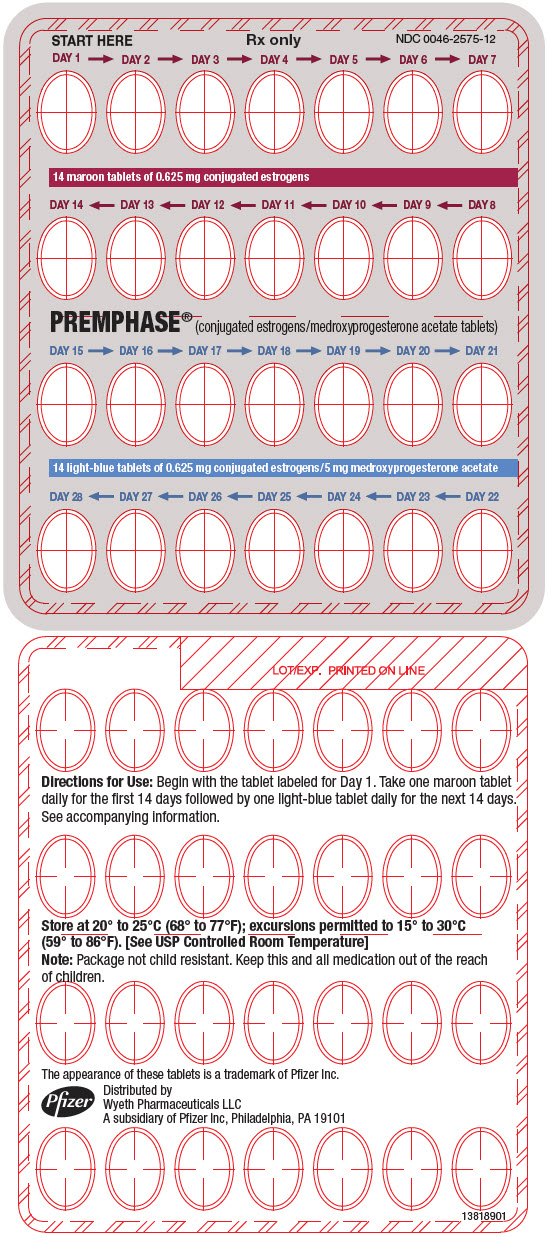
10PRINCIPAL DISPLAY PANEL - 28 Tablet 0.625 mg / 5 mg Blister Pack
START HERE
DAY 1 → DAY 2 → DAY 3 → DAY 4 → DAY 5 → DAY 6 → DAY 7
14 maroon tablets of 0.625 mg conjugated estrogens
DAY 14 ← DAY 13 ← DAY 12 ← DAY 11 ← DAY 10 ← DAY 9 ← DAY 8
PREMPHASE
DAY 15 → DAY 16 → DAY 17 → DAY 18 → DAY 19 → DAY 20 → DAY 21
14 light-blue tablets of 0.625 mg conjugated estrogens/5 mg medroxyprogesterone acetate
DAY 28 ← DAY 27 ← DAY 26 ← DAY 25 ← DAY 24 ← DAY 23 ← DAY 22
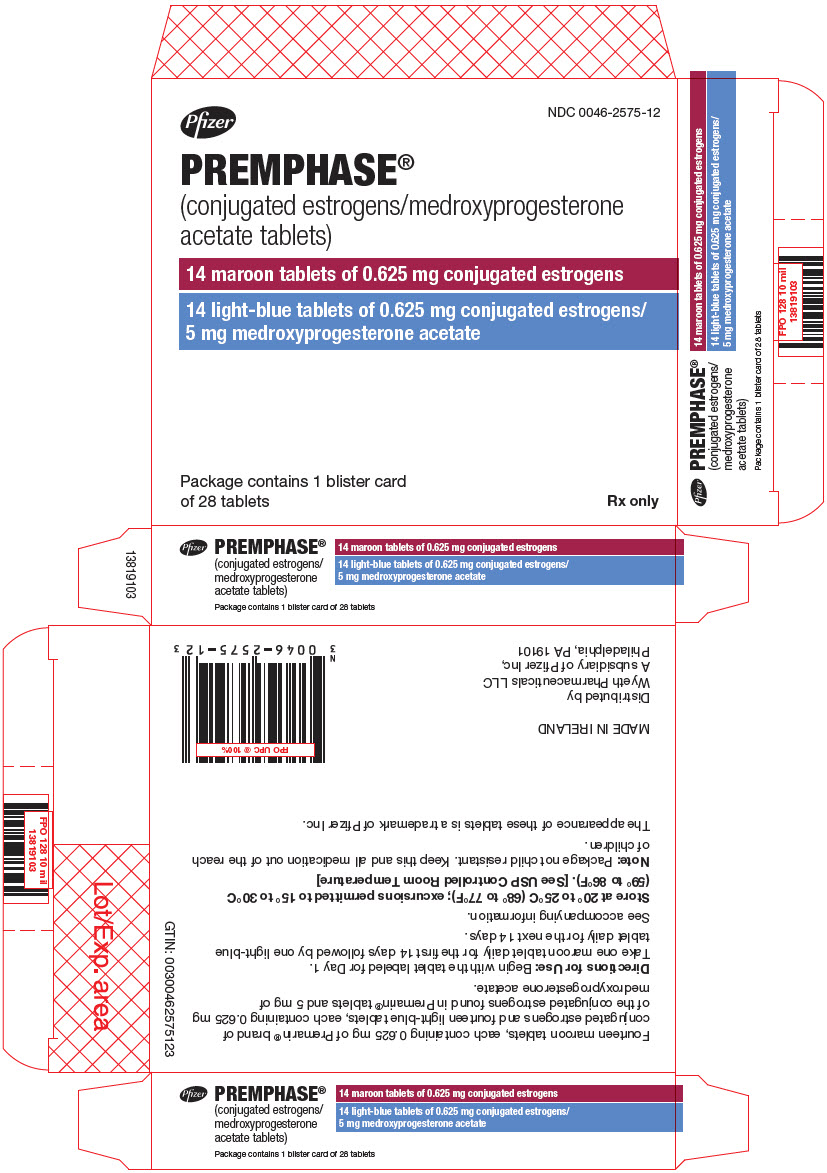
11PRINCIPAL DISPLAY PANEL - Kit Carton
NDC 0046-2575-12
Pfizer
PREMPHASE
14 maroon tablets of 0.625 mg conjugated estrogens
14 light-blue tablets of 0.625 mg conjugated estrogens/
Package contains 1 blister card
Rx only
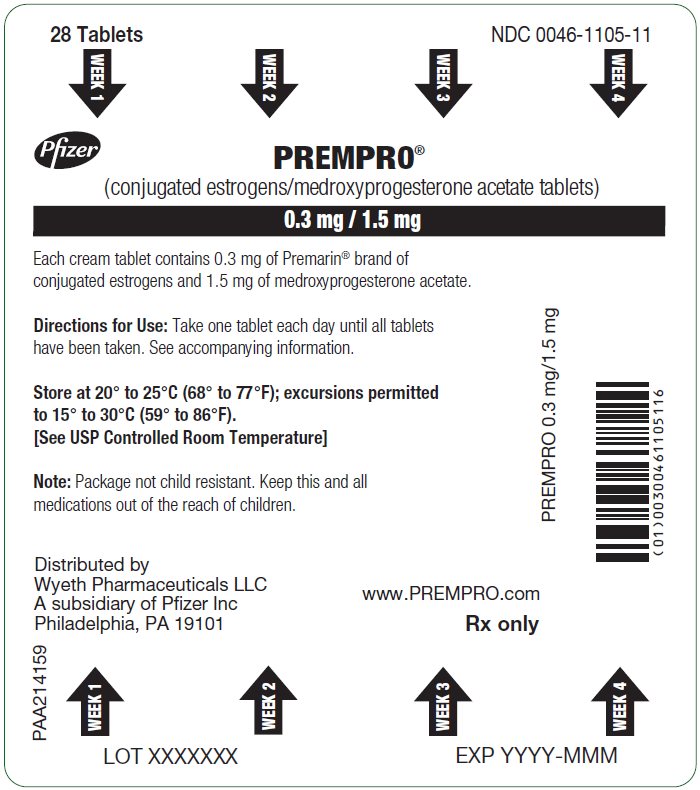
12PRINCIPAL DISPLAY PANEL - 0.3 mg / 1.5 mg Blister Card
28 Tablets
WEEK 1
Pfizer
PREMPRO
0.3 mg / 1.5 mg
Each cream tablet contains 0.3 mg of Premarin
Directions for Use: Take one tablet each day until all tablets
Store at 20° to 25°C (68° to 77°F); excursions permitted
Note: Package not child resistant. Keep this and all
PREMPRO 0.3 mg/1.5 mg
Distributed by
www.PREMPRO.com
Rx only
PAA224096
WEEK 1
LOT XXXXXXX
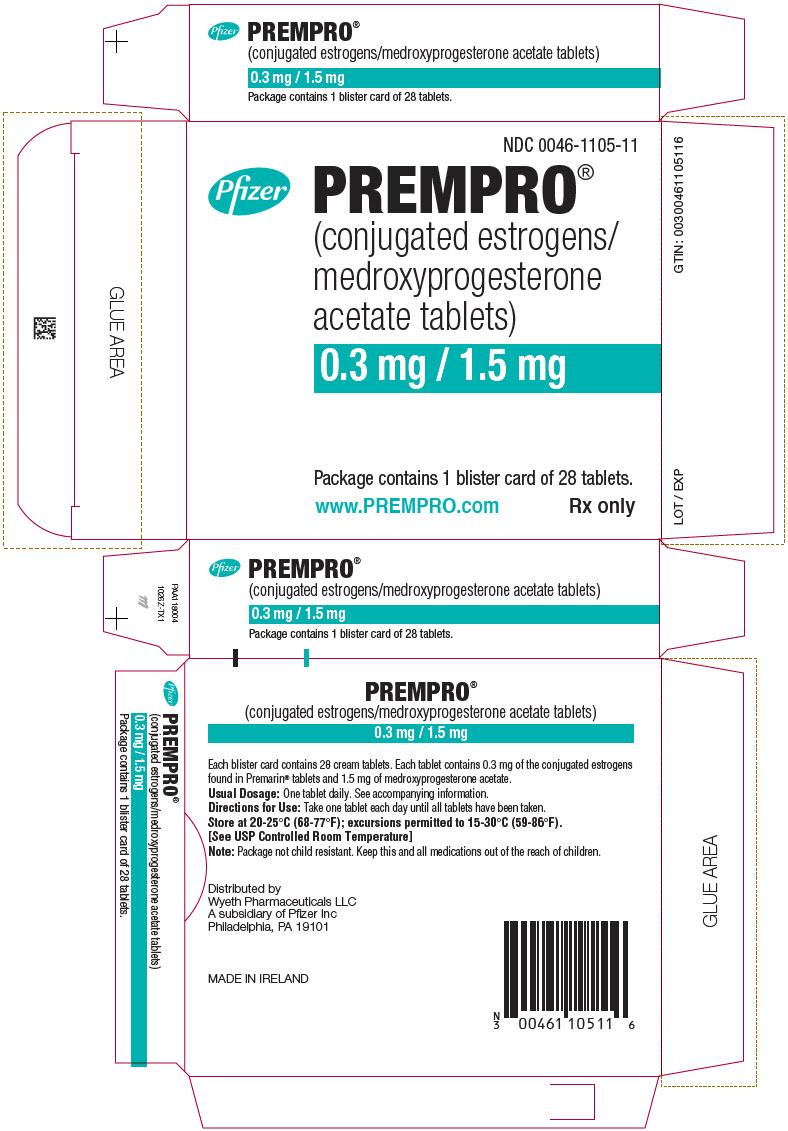
13PRINCIPAL DISPLAY PANEL - 0.3 mg / 1.5 mg Blister Card Carton
NDC 0046-1105-11
Pfizer
PREMPRO
0.3 mg / 1.5 mg
Package contains 1 blister card of 28 tablets.
www.PREMPRO.com
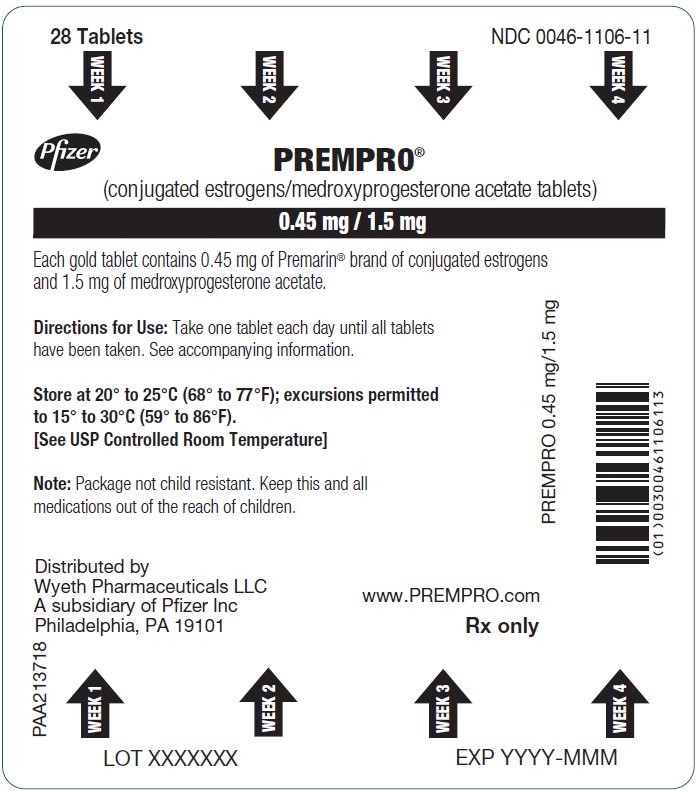
14PRINCIPAL DISPLAY PANEL - 0.45 mg / 1.5 mg Blister Card
28 Tablets
WEEK 1
Pfizer
PREMPRO
0.45 mg / 1.5 mg
Each gold tablet contains 0.45 mg of Premarin
Directions for Use: Take one tablet each day until all tablets
Store at 20° to 25°C (68° to 77°F); excursions permitted
Note: Package not child resistant. Keep this and all
PREMPRO 0.45 mg/1.5 mg
Distributed by
www.PREMPRO.com
Rx only
PAA224094
WEEK 1
LOT XXXXXXX
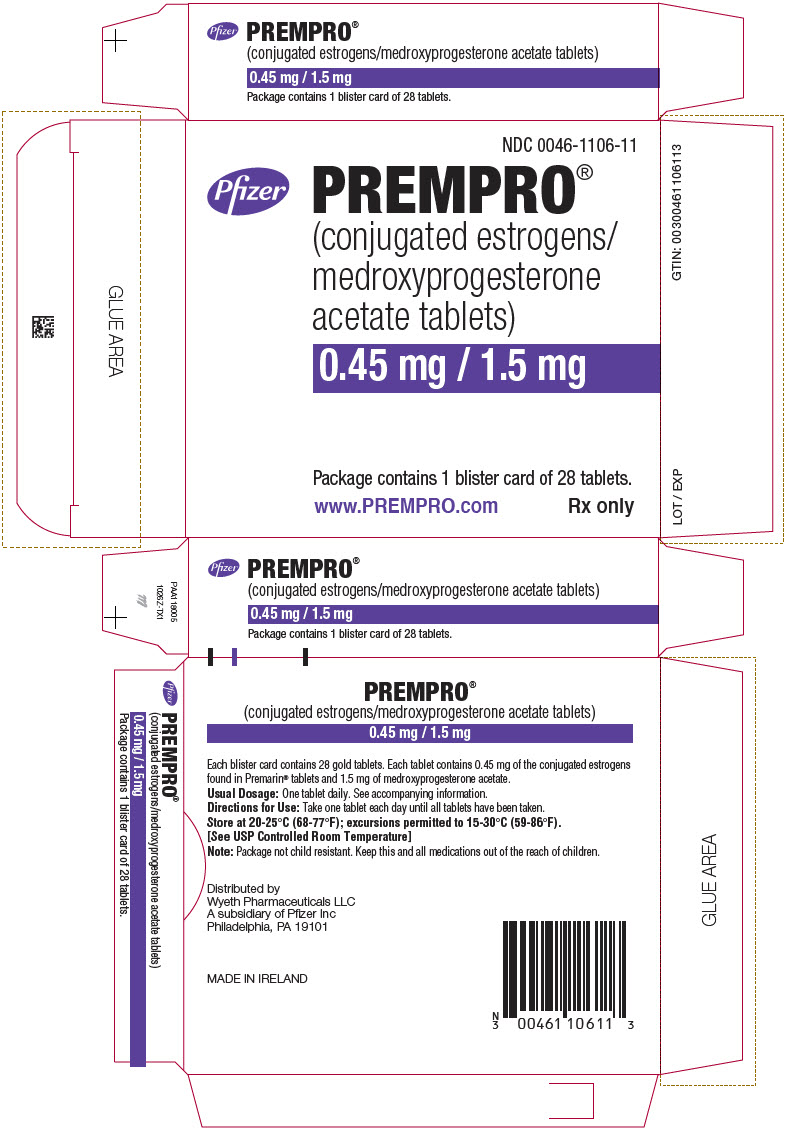
15PRINCIPAL DISPLAY PANEL - 0.45 mg / 1.5 mg Blister Card Carton
NDC 0046-1106-11
Pfizer
PREMPRO
0.45 mg / 1.5 mg
Package contains 1 blister card of 28 tablets.
www.PREMPRO.com
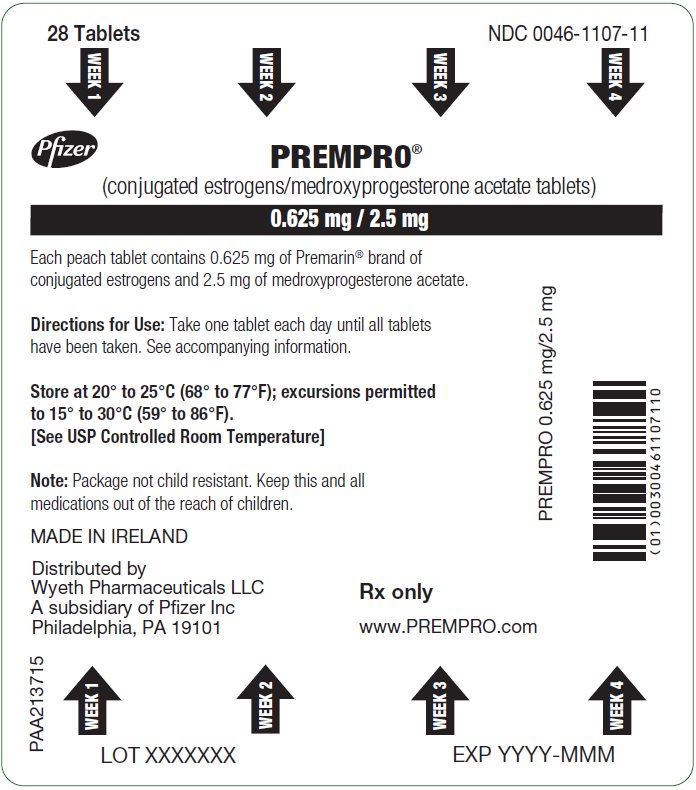
16PRINCIPAL DISPLAY PANEL - 0.625 mg / 2.5 mg Tablet Blister Card
28 Tablets
WEEK 1
Pfizer
PREMPRO
0.625 mg / 2.5 mg
Each peach tablet contains 0.625 mg of Premarin
Directions for Use: Take one tablet each day until all tablets
Store at 20° to 25°C (68° to 77°F); excursions permitted
Note: Package not child resistant. Keep this and all
MADE IN IRELAND
PREMPRO 0.625 mg/2.5 mg
Distributed by
Rx only
www.PREMPRO.com
PAA224092
WEEK 1
LOT XXXXXXX
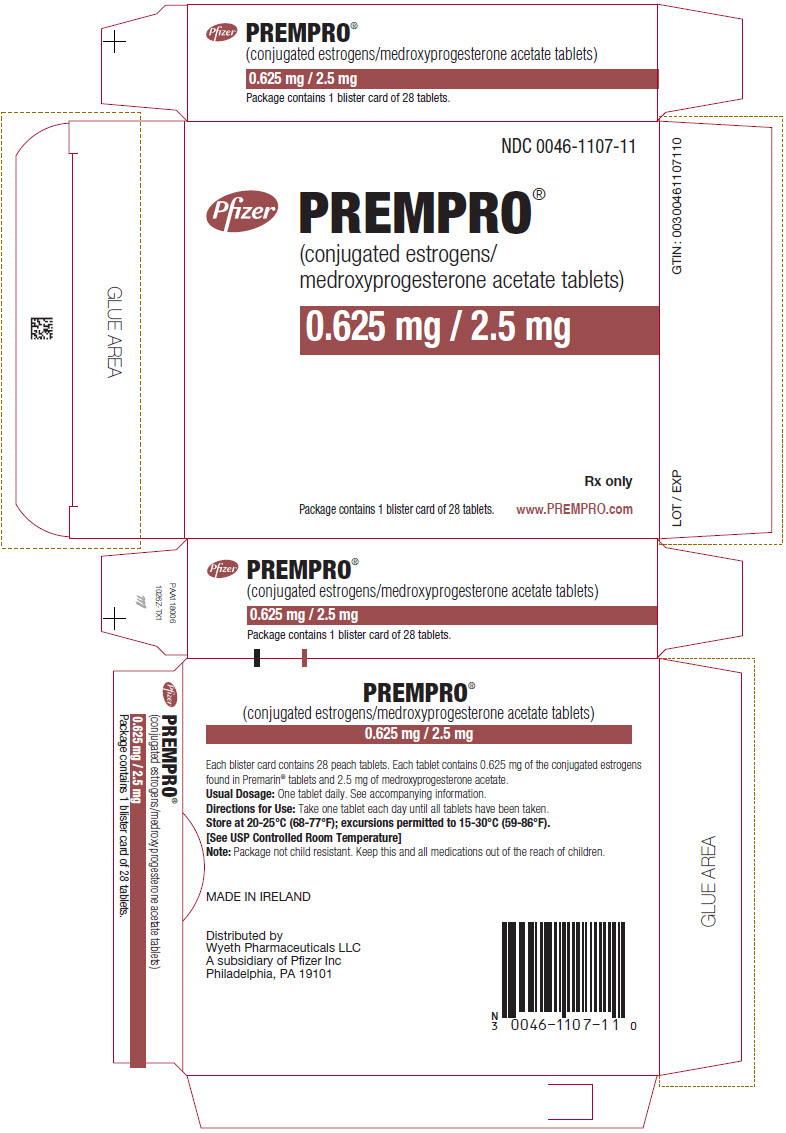
17PRINCIPAL DISPLAY PANEL - 0.625 mg / 2.5 mg Tablet Blister Card Carton
NDC 0046-1107-11
Pfizer
0.625 mg / 2.5 mg
Rx only
Package contains 1 blister card of 28 tablets.
18PRINCIPAL DISPLAY PANEL - 0.625 mg / 5 mg Tablet Blister Card
28 Tablets
WEEK 1
Pfizer
PREMPRO
Rx only
0.625 mg / 5 mg
Each light-blue tablet contains 0.625 mg of Premarin
Directions for Use: Take one tablet each day until all tablets
Store at 20° to 25°C (68° to 77°F); excursions permitted
Note: Package not child resistant. Keep this and all
MADE IN IRELAND
PREMPRO 0.625 mg/5 mg
Distributed by
www.PREMPRO.com
PAA224000
WEEK 1
LOT XXXXXXX

19PRINCIPAL DISPLAY PANEL - 0.625 mg / 5 mg Tablet Blister Card Carton
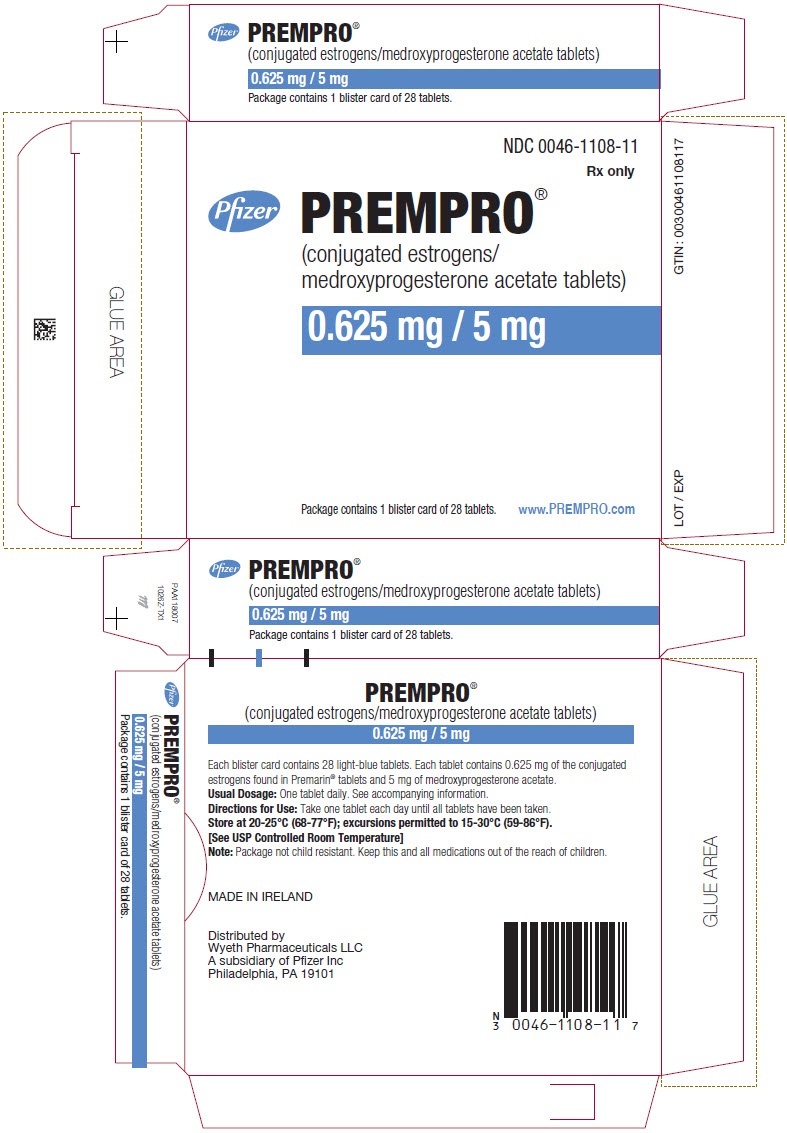
NDC 0046-1108-11
Rx only
Pfizer
0.625 mg / 5 mg
Package contains 1 blister card of 28 tablets.