Brand Name
Nayzilam
Generic Name
Midazolam
View Brand Information FDA approval date: June 20, 2000
Classification: Benzodiazepine
Form: Injection, Spray, Syrup
What is Nayzilam (Midazolam)?
Midazolam HCl Syrup is indicated for use in pediatric patients for sedation, anxiolysis and amnesia prior to diagnostic, therapeutic or endoscopic procedures or before induction of anesthesia. Midazolam HCl syrup is intended for use in monitored settings only and not for chronic or home use. WARNINGS MIDAZOLAM HCL SYRUP MUST BE USED AS SPECIFIED IN THE LABEL. Midazolam is associated with a high incidence of partial or complete impairment of recall for the next several hours. CLINICAL PHARMACOLOGY.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Nayzilam (midazolam)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death
- The use of benzodiazepines, including NAYZILAM, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing NAYZILAM and throughout treatment, assess each patient's risk for abuse, misuse, and addiction
- The continued use of benzodiazepines may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Although NAYZILAM is indicated only for intermittent use
1INDICATIONS AND USAGE
NAYZILAM is indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (i.e., seizure clusters, acute repetitive seizures) that are distinct from a patient's usual seizure pattern in patients with epilepsy 12 years of age and older.
2DOSAGE FORMS AND STRENGTHS
NAYZILAM is supplied as a single-dose nasal spray unit containing 5 mg of midazolam in 0.1 mL solution.
3CONTRAINDICATIONS
NAYZILAM is contraindicated in patients with:
- Known hypersensitivity to midazolam.
- Acute narrow-angle glaucoma
4ADVERSE REACTIONS
The following serious adverse reactions are discussed in more detail in other sections of the labeling:
- Risks from Concomitant Use with Opioids
- Abuse, Misuse, and Addiction
- Dependence and Withdrawal Reactions After Use of NAYZILAM More Frequently Than Recommended
- Risks of Cardiorespiratory Adverse Reactions
- CNS Depression from Concomitant Use with Other CNS Depressants or Moderate or Strong CYP3A4 Inhibitors
- Suicidal Behavior and Ideation
- Impaired Cognitive Function
- Glaucoma
- Neonatal Sedation and Withdrawal Syndrome
- Other Adverse Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
NAYZILAM was studied for the outpatient treatment of a single seizure cluster in 292 adult and adolescent patients with epilepsy (Study 1)
Table 2 lists the adverse reactions occurring in 2% or more of the NAYZILAM-treated patients and at a rate greater than the placebo-treated patients in the Comparative Phase of Study 1.
For patients who experienced a decrease in peripheral oxygen saturation in the Test Dose Phase of Study 1, the decreases were generally transitory. Two patients (one with a history of sleep apnea and one with intercurrent seizure) with decreases in peripheral oxygen saturation in the Test Dose Phase required therapeutic supplemental oxygen.
5OVERDOSAGE
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, excitement, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway maintenance. Flumazenil, a specific benzodiazepine receptor antagonist indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures, particularly in the context of mixed overdosage with drugs that increase seizure risk (e.g., tricyclic and tetracyclic antidepressants) and in patients with long-term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus). If the decision is made to use flumazenil, it should be used as an adjunct to, not as a substitute for, supportive management of benzodiazepine overdosage. See the flumazenil injection Prescribing Information.
Consider contacting the Poison Help line (1-800-222-1222) or medical toxicologist for additional overdosage management recommendations.
6DESCRIPTION
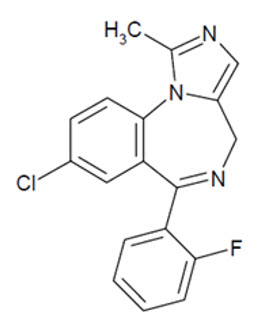
NAYZILAM contains midazolam, a compound of the benzodiazepine class. Midazolam is chemically designated as 8-Chloro-6-(ο-fluorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepine, and it has the following structure:
The empirical formula is C
NAYZILAM nasal spray is a clear, colorless to yellowish colored liquid. Each single-dose NAYZILAM unit is for nasal administration and delivers 5 mg of midazolam in 0.1 mL of solution containing ethanol; PEG-6 methyl ether; polyethylene glycol 400; propylene glycol; and purified water.
The pH range of solution is approximately 5.0 to 9.0.
7CLINICAL STUDIES
The effectiveness of NAYZILAM for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (i.e., seizure clusters, acute repetitive seizures) that are distinct from a patient's usual seizure pattern in patients with epilepsy 12 years of age and older was established in a randomized, double-blind, placebo-controlled trial (Study 1; NCT 01390220).
Study 1 enrolled patients with epilepsy on a stable regimen of antiepileptic drugs who were identified by their physicians as having intermittent, stereotypic episodes of frequent seizure activity that were distinct from the patient's usual seizure pattern.
Study 1 was conducted in two phases: an open-label Test Dose Phase followed by a randomized, double-blind, placebo-controlled, Comparative Phase. In the Test Dose Phase, tolerability was assessed in 292 patients who, in the absence of a seizure, received two 5 mg doses of NAYZILAM (10 mg total dosage) separated by 10 minutes. Patients were excluded from participation in the Comparative Phase if they failed to meet pre-defined blood pressure, heart rate, sedation, electrocardiogram, and peripheral oxygen saturation criteria.
In the Comparative Phase, 201 patients treated a single seizure cluster episode in an outpatient setting with either a blinded dose of NAYZILAM 5 mg (134 patients) or placebo (67 patients). If the seizure activity persisted or recurred, patients in both groups had the option to receive a subsequent unblinded dose of NAYZILAM 5 mg to be used between 10 minutes and 6 hours after administration of the initial blinded dose of study drug.
The primary efficacy endpoint for Study 1 was treatment success, defined as the termination of seizures within 10 minutes after the initial blinded dose of study drug and the absence of a recurrence of seizures within 6 hours of the initial blinded dose of study drug. A statistically significantly higher percentage of NAYZILAM-treated patients met the primary efficacy endpoint, as shown in Table 4.
Numerical differences in favor of NAYZILAM were observed on each of the components of the treatment success responder definition; termination of seizure(s) within 10 minutes after initial dose of study drug (80.6 versus 70.1%) and the absence of seizure recurrence between 10 minutes and 6 hours after the initial dose of study drug (58.2 versus 37.3%).
Study 1 also evaluated the occurrence and time to next seizure after the initial blinded dose of study drug. A smaller proportion of NAYZILAM-treated patients experienced the next seizure within 24 hours after the initial blinded dose of study drug (37.3% versus 46.3%). NAYZILAM-treated patients experienced a statistically longer time-to-next-seizure than the placebo group (Figure 1).
FIGURE 1: Kaplan-Meier Analysis of Time-to-Next-Seizure (Study 1)
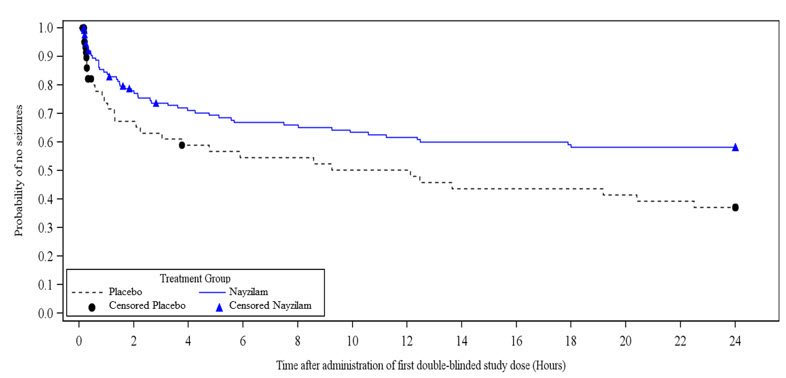
Analysis by gender revealed no substantial differences in treatment response. Informative subgroup analyses by age and race were not possible because of the small percentage of patients less than 18 years of age or 65 years of age or greater, and of non-White patients in the study.
8PATIENT COUNSELING INFORMATION
Advise patients and caregivers to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
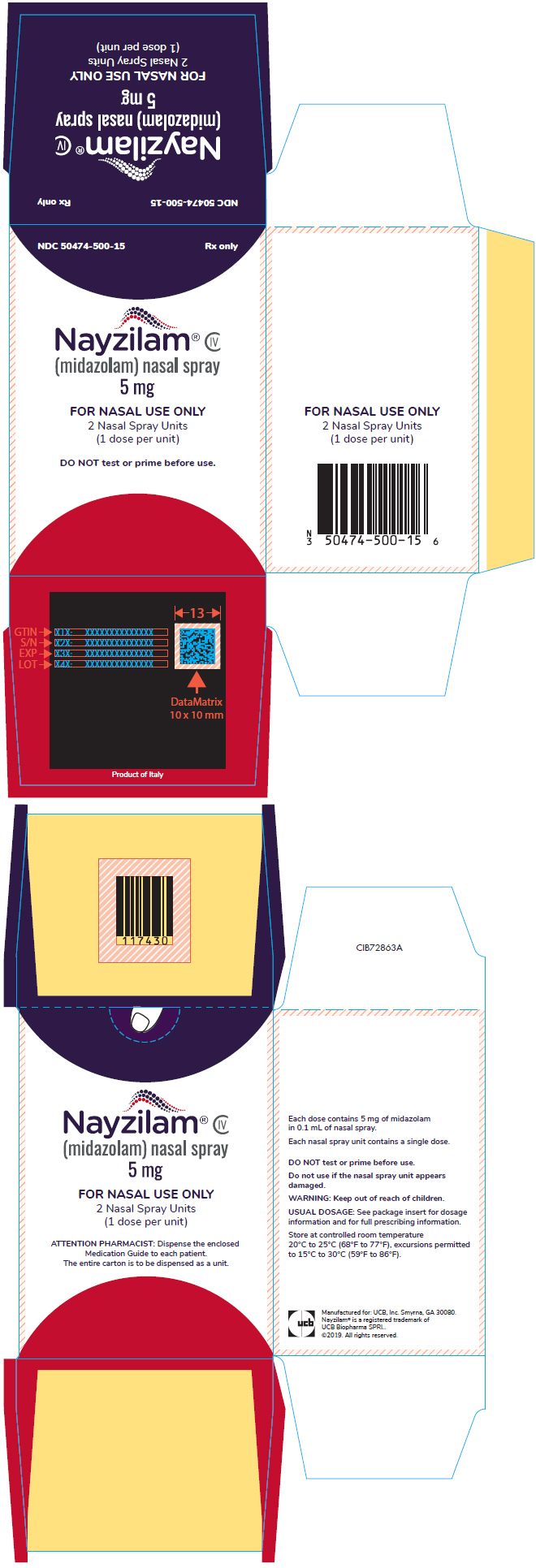
9PRINCIPAL DISPLAY PANEL - 5 mg Vial Blister Pack Carton
NDC 50474-500-15
Nayzilam
FOR NASAL USE ONLY
DO NOT test or prime before use.