Brand Name
Yorvipath
Generic Name
Palopegteriparatide
View Brand Information FDA approval date: August 09, 2024
Classification: Parathyroid Hormone Analog
Form: Injection
What is Yorvipath (Palopegteriparatide)?
YORVIPATH is indicated for the treatment of hypoparathyroidism in adults. YORVIPATH is a parathyroid hormone analog (PTH) indicated for the treatment of hypoparathyroidism in adults. Limitations of Use : Not studied for acute post-surgical hypoparathyroidism. Titration scheme only evaluated in adults who first achieved an albumin-corrected serum calcium of at least.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Yorvipath (Palopegteriparatide)
1INDICATIONS AND USAGE
YORVIPATH is indicated for the treatment of hypoparathyroidism in adults.
2DOSAGE FORMS AND STRENGTHS
Injection: Clear, colorless solution in single-patient-use prefilled pens in three presentations
Table 2 displays the YORVIPATH prefilled pen presentations, strengths, labeled doses, and deliverable dose ranges.
3CONTRAINDICATIONS
YORVIPATH is contraindicated in patients with severe hypersensitivity to palopegteriparatide or to any of its excipients. Hypersensitivity reactions, including anaphylaxis, angioedema, and urticaria, have been observed with parathyroid hormone (PTH) analogs.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Risk of Unintended Changes in Serum Calcium Levels Related to Number of Daily Injections
- Serious Hypercalcemia
- Serious Hypocalcemia
- Potential Risk of Osteosarcoma
- Orthostatic Hypotension
- Risk of Digoxin Toxicity with Concomitant Use of Digitalis Compounds
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The phase 3 trial included 82 subjects with hypoparathyroidism with a median YORVIPATH treatment duration of 182 days (Study 1)
Adverse reactions associated with YORVIPATH in Study 1 during the 26-week blinded period (incidence ≥5% and occurring ≥2% more frequently than placebo) are shown in Table 3.
5OVERDOSAGE
Accidental overdose of YORVIPATH may cause hypercalcemia that can be severe and require medical intervention. One subject in Study 1 accidentally received approximately 3-fold the prescribed dose of YORVIPATH for more than 7 consecutive days and developed albumin-corrected serum calcium as high as 16.1 mg/dL, requiring hospitalization.
6DESCRIPTION
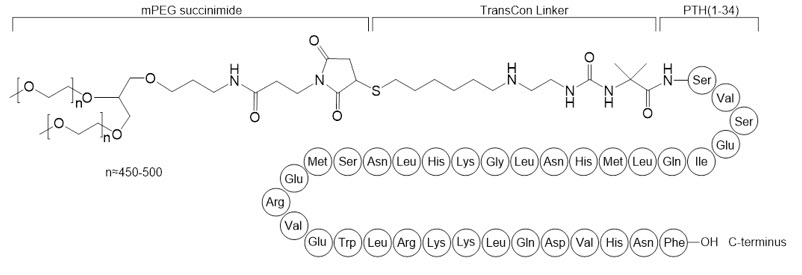
YORVIPATH (palopegteriparatide injection) is a parathyroid hormone analog (PTH(1-34)). Palopegteriparatide is a prodrug of teriparatide (PTH(1-34)) consisting of PTH(1-34) transiently conjugated to an inert carrier via a proprietary TransCon Linker. PTH(1-34) is identical to the 34 N-terminal amino acids (the biologically active region) of the 84-amino acid human parathyroid hormone. The carrier is a branched 40 kDa (2×20 kDa) methoxypolyethylene glycol (mPEG) moiety. The average molecular weight of palopegteriparatide is approximately 47.4 kDa.
The structure of palopegteriparatide drug substance is shown in Figure 2. The theoretical molecular formula is C
Figure 2: Structure of Palopegteriparatide
YORVIPATH is a sterile, clear, and colorless solution in a glass cartridge which is pre-assembled in a single-patient-use prefilled pen for subcutaneous administration. The prefilled pen is co-packaged with disposable needles to administer 14 doses of YORVIPATH. YORVIPATH is available in three presentations containing 0.56 mL, 0.98 mL, or 1.4 mL of YORVIPATH solution, and each pen presentation can deliver one of three distinct doses for 14 days of therapy.
Each mL of YORVIPATH solution contains 3456 mcg of palopegteriparatide, equivalent to 300 mcg of teriparatide (PTH(1-34)), and the following inactive ingredients: 41.7 mg mannitol, 2.5 mg metacresol, 0.13 mg sodium hydroxide, 1.18 mg succinic acid, and water for injection. YORVIPATH has a pH of 3.7 to 4.3.
Each pen of 0.56 mL contains 168 mcg teriparatide equivalent to 1935 mcg palopegteriparatide.
Each pen of 0.98 mL contains 294 mcg teriparatide equivalent to 3387 mcg palopegteriparatide.
Each pen of 1.4 mL contains 420 mcg teriparatide equivalent to 4838 mcg palopegteriparatide.
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
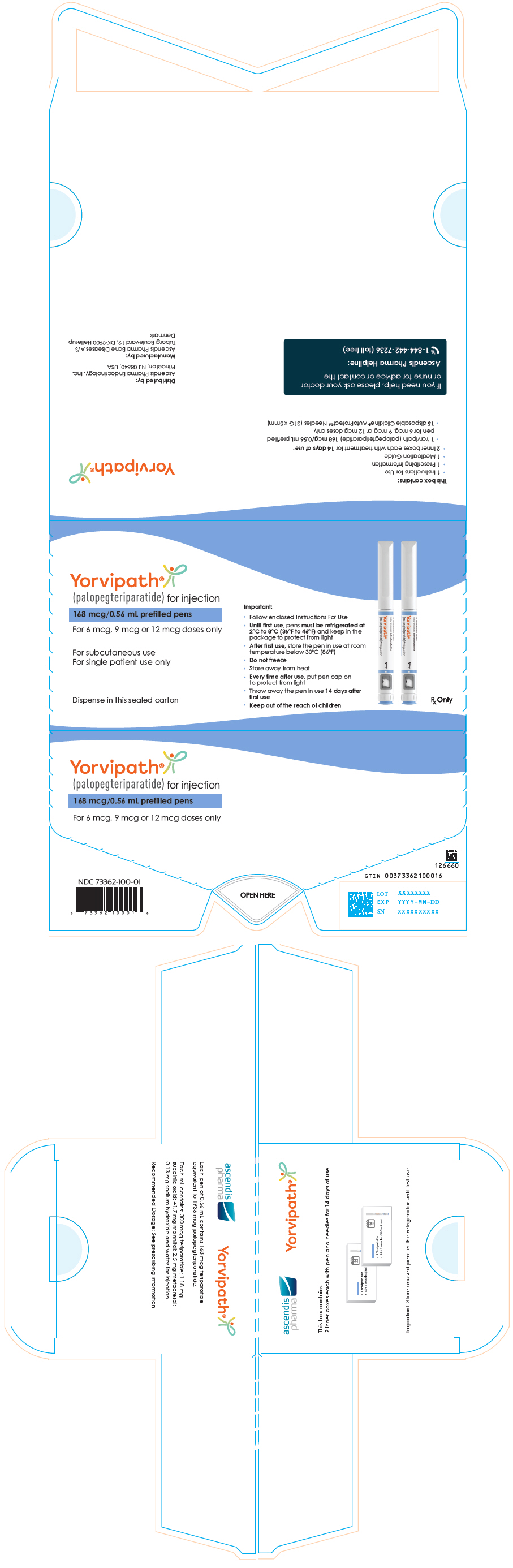
8PRINCIPAL DISPLAY PANEL - 168 mcg/0.56 mL Pen Carton
Yorvipath
168 mcg/0.56 mL prefilled pens
For 6 mcg, 9 mcg or 12 mcg doses only
For subcutaneous use
Dispense in this sealed carton
Important:
- Follow enclosed Instructions For Use
- Until first use, pens must be refrigerated at
- After first use, store the pen in use at room
- Do not freeze
- Store away from heat
- Every time after use, put pen cap on
- Throw away the pen in use 14 days after
- Keep out of the reach of children
Rx Only
9PRINCIPAL DISPLAY PANEL - 294 mcg/0.98 mL Pen Carton
Yorvipath
294 mcg/0.98 mL prefilled pens
For 15 mcg, 18 mcg or 21 mcg doses only
For subcutaneous use
Dispense in this sealed carton
Important:
- Follow enclosed Instructions For Use
- Until first use, pens must be refrigerated at
- After first use, store the pen in use at room
- Do not freeze
- Store away from heat
- Every time after use, put pen cap on
- Throw away the pen in use 14 days after
- Keep out of the reach of children
Rx Only

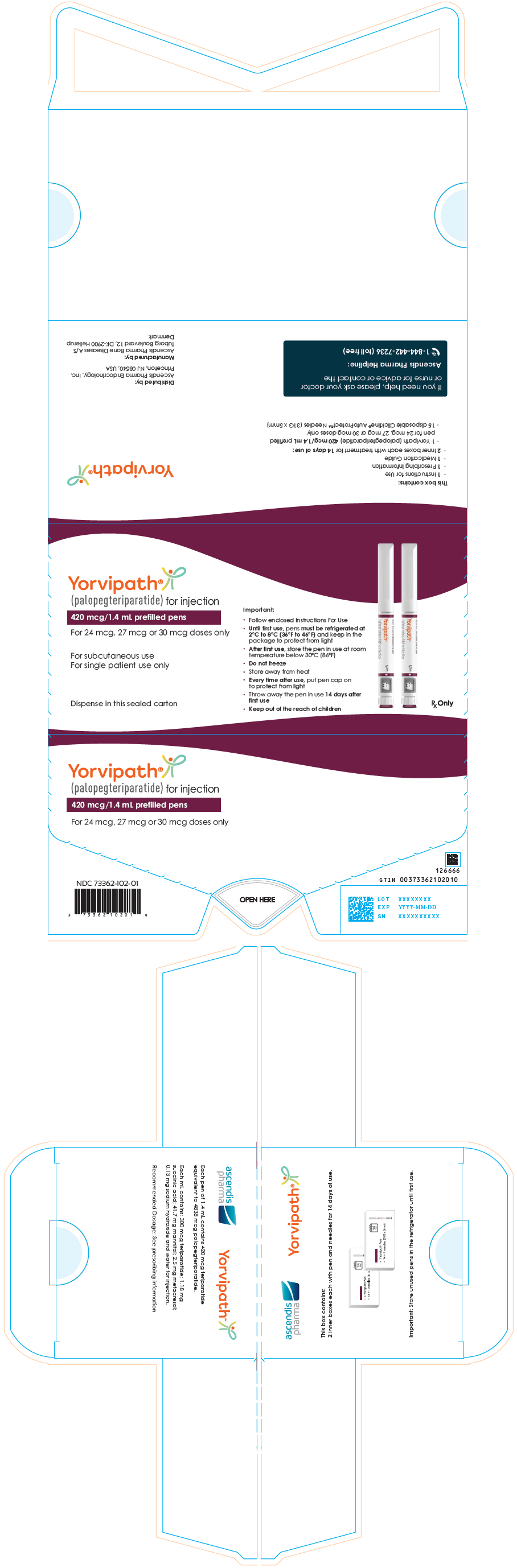
10PRINCIPAL DISPLAY PANEL - 420 mcg/1.4 mL Pen Carton
Yorvipath
420 mcg/1.4 mL prefilled pens
For 24 mcg, 27 mcg or 30 mcg doses only
For subcutaneous use
Dispense in this sealed carton
Important:
- Follow enclosed Instructions For Use
- Until first use, pens must be refrigerated at
- After first use, store the pen in use at room
- Do not freeze
- Store away from heat
- Every time after use, put pen cap on
- Throw away the pen in use 14 days after
- Keep out of the reach of children
Rx Only