Brand Name
Niktimvo
Generic Name
Axatilimab-Csfr
View Brand Information FDA approval date: August 14, 2024
Classification: Colony Stimulating Factor-1 Receptor Blocker
Form: Injection
What is Niktimvo (Axatilimab-Csfr)?
NIKTIMVO is indicated for the treatment of chronic graft-versus-host disease after failure of at least two prior lines of systemic therapy in adult and pediatric patients weighing at least 40 kg. NIKTIMVO is a colony stimulating factor-1 receptor -blocking antibody indicated for the treatment of chronic graft-versus-host disease after failure of at least two prior lines of systemic therapy in adult and pediatric patients weighing at least 40 kg.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Brand Information
NIKTIMVO (axatilimab-csfr)
1INDICATIONS AND USAGE
NIKTIMVO is indicated for the treatment of chronic graft-versus-host disease (cGVHD) after failure of at least two prior lines of systemic therapy in adult and pediatric patients weighing at least 40 kg.
2DOSAGE FORMS AND STRENGTHS
NIKTIMVO injection is a slightly opalescent, pale brownish yellow solution available as:
- 9 mg/0.18 mL in a single-dose vial.
- 22 mg/0.44 mL in a single-dose vial.
- 50 mg/mL in a single-dose vial.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following adverse reactions are described elsewhere in the labeling.
- Infusion-Related Reactions
4.1Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Chronic Graft-Versus-Host Disease
The safety of NIKTIMVO was evaluated in 79 adult and pediatric patients with cGVHD treated with NIKTIMVO 0.3 mg/kg intravenously every 2 weeks in the AGAVE‑201 trial
Serious adverse reactions occurred in 44% of patients who received NIKTIMVO. Serious adverse reactions in more than 2 patients included infection (pathogen unspecified), viral infection, and respiratory failure. Permanent discontinuation of NIKTIMVO due to an adverse reaction occurred in 10% of patients and dose reduction due to adverse reaction occurred in 8% of patients. Dose interruptions due to an adverse reaction occurred in 44% of patients. The adverse reactions leading to dose interruption in more than 2 patients were viral infection, infection (pathogen unspecified), bacterial infection, musculoskeletal pain, and pyrexia.
The most common (≥ 15%) adverse reactions, including laboratory abnormalities, were increased AST, infection (pathogen unspecified), increased ALT, decreased phosphate, decreased hemoglobin, viral infection, increased gamma glutamyl transferase (GGT), musculoskeletal pain, increased lipase, fatigue, increased amylase, increased calcium, increased CPK, increased ALP, nausea, headache, diarrhea, cough, bacterial infection, pyrexia, and dyspnea.
Table 2 summarizes the nonlaboratory adverse reactions in AGAVE-201.
Clinically relevant adverse reactions in < 10% of patients who received NIKTIMVO included:
- Eye disorders: periorbital edema
- Skin and subcutaneous skin disorders: pruritus
- Vascular disorders: hypertension
Table 3 summarizes the laboratory abnormalities in AGAVE-201.
Immunogenicity: Anti-Drug Antibody–Associated Adverse Reactions
In 276 patients with cGVHD who received NIKTIMVO in clinical trials, among the patients who developed anti-drug antibodies (ADAs), hypersensitivity reactions occurred in 26% (13/50) of patients with neutralizing antibodies (NAb) and in 4% (2/45) of those without NAb [see Clinical Pharmacology (.
5DESCRIPTION
Axatilimab-csfr is a CSF-1R–blocking antibody. Axatilimab-csfr is a humanized IgG4 (kappa light chain) monoclonal antibody produced in Chinese hamster ovary cells. Axatilimab-csfr has an approximate molecular weight of 150 kDa.
6CLINICAL STUDIES
The efficacy of NIKTIMVO was evaluated in AGAVE-201 (NCT04710576), a randomized, open-label, multicenter study in adult and pediatric patients with recurrent or refractory cGVHD who had received at least 2 lines of systemic therapy and required additional treatment. Patients with platelet count ≥ 50 × 10
The efficacy of NIKTIMVO was based on overall response rate (ORR) through Cycle 7 Day 1, where overall response included complete response or partial response according to the 2014 NIH Consensus Development Project on Response Criteria. The ORR results from AGAVE-201 for the 0.3 mg/kg every 2 weeks dosage regimen are presented in Table 5. The median time to first response was 1.5 months (range, 0.9 to 5.1 months). The median duration of response, calculated from first response to progression, death, or new systemic therapies for cGVHD, was 1.9 months (95% CI: 1.6, 3.5). In patients who achieved response, no death or new systemic therapy initiation occurred in 60% (95% CI: 43, 74) of patients for at least 12 months since response.
ORR results were supported by exploratory analyses of patient-reported symptom bother which showed at least a 7-point decrease in the modified Lee Symptom Scale score through Cycle 7 Day 1 in 56% (95% CI: 44, 67) of patients.
7HOW SUPPLIED/STORAGE AND HANDLING
NIKTIMVO (axatilimab-csfr) injection is a slightly opalescent, pale brownish yellow solution. It is supplied in a carton containing one single-dose vial either as:
- 9 mg/0.18 mL (NDC 50881-034-12)
- 22 mg/0.44 mL (NDC 50881-023-11)
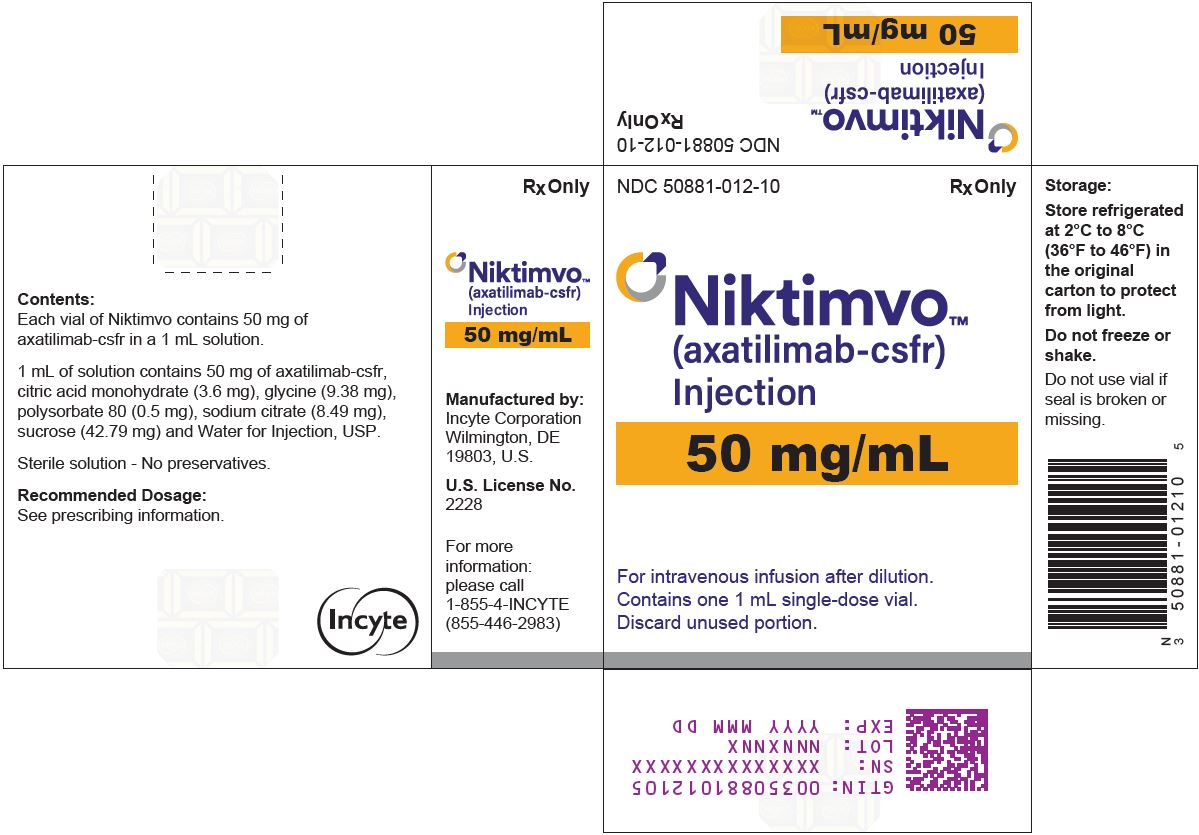
- 50 mg/mL (NDC 50881-012-10)
Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not freeze or shake.
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Licensed from:
NIKTIMVO and the NIKTIMVO logo are trademarks of Incyte.
9NIKTIMVO 50 MG/ML CARTON
NDC 50881-012-10
NIKTIMVO
For intravenous infusion after dilution.
10NIKTIMVO 9 MG/0.18ML CARTON
NDC 50881-034-12
NIKTIMVO
For intravenous infusion after dilution.

11NIKTIMVO 22 MG/0.44ML CARTON
NDC 50881-023-11
NIKTIMVO
For intravenous infusion after dilution.
