Generic Name
Cyclobenzaprine
Brand Names
AMRIX, Tonmya, Fexmid
FDA approval date: April 04, 2006
Classification: Muscle Relaxant
Form: Tablet, Capsule
What is AMRIX (Cyclobenzaprine)?
Cyclobenzaprine hydrochloride extended-release capsules are indicated as an adjunct to rest and physical therapy for relief of muscle spasm associated with acute, painful musculoskeletal conditions. Improvement is manifested by relief of muscle spasm and its associated signs and symptoms, namely, pain, tenderness, and limitation of motion. Limitations of Use: Cyclobenzaprine hydrochloride extended-release capsules should be used only for short periods because adequate evidence of effectiveness for more prolonged use is not available and because muscle spasm associated with acute, painful musculoskeletal conditions is generally of short duration and specific therapy for longer periods is seldom warranted. Cyclobenzaprine hydrochloride extended-release capsules have not been found effective in the treatment of spasticity associated with cerebral or spinal cord disease or in children with cerebral palsy.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
AMRIX (Cyclobenzaprine Hydrochloride)
1INDICATIONS AND USAGE
AMRIX
Limitations of Use:
- AMRIX should be used only for short periods (up to two or three weeks) because adequate evidence of effectiveness for more prolonged use is not available and because muscle spasm associated with acute, painful musculoskeletal conditions is generally of short duration and specific therapy for longer periods is seldom warranted.
- AMRIX has not been found effective in the treatment of spasticity associated with cerebral or spinal cord disease or in children with cerebral palsy.
2DOSAGE AND ADMINISTRATION
The recommended adult dose for most patients is one (1) AMRIX 15 mg capsule taken once daily. Some patients may require up to 30 mg/day, given as one (1) AMRIX 30 mg capsule taken once daily or as two (2) AMRIX 15 mg capsules taken once daily.
- It is recommended that doses be taken at approximately the same time each day.
- Use of AMRIX for periods longer than two or three weeks is not recommended
Instruct patients to swallow AMRIX capsules intact. Alternatively, the contents of the AMRIX capsule may be sprinkled over applesauce and then swallowed. This method is appropriate only for patients able to reliably swallow the applesauce without chewing. Other foods have not been tested and should not be substituted for applesauce. Instruct the patient to:
- Sprinkle the contents of the capsule onto a tablespoon of applesauce and consume immediately without chewing.
- Rinse the mouth to ensure all of the contents have been swallowed.
- Discard any unused portion of the AMRIX capsules after the contents have been sprinkled on applesauce.
3DOSAGE FORMS AND STRENGTHS
Extended-release capsules in the following strengths:
- 15 mg: Capsules are orange/orange and are embossed in blue ink with “15 mg” on the body, and Cephalon “C” logo, “Cephalon,” and a dashed band on the cap.
- 30 mg: Capsules are blue/red and are embossed in white ink with “30 mg” on the body, and Cephalon “C” logo, “Cephalon,” and a dashed band on the cap.
4CONTRAINDICATIONS
- Hypersensitivity to any component of this product. Adverse reactions may include anaphylactic reaction, urticaria, facial and/or tongue swelling, or pruritus. Discontinue AMRIX if a hypersensitivity reaction is suspected.
- Concomitant use of monoamine oxidase (MAO) inhibitors or within 14 days after their discontinuation. Hyperpyretic crisis seizures and deaths have occurred in patients receiving cyclobenzaprine (or structurally similar tricyclic antidepressants) concomitantly with MAO inhibitor drugs.
- During the acute recovery phase of myocardial infarction, and in patients with arrhythmias, heart block or conduction disturbances, or congestive heart failure.
- Hyperthyroidism.
5ADVERSE REACTIONS
The following clinically significant reactions are described in greater detail, in other sections.
- Serotonin Syndrome
- Adverse Cardiovascular Effects [see Warnings and Precautions (
5.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described below reflect exposure to AMRIX in 253 patients in 2 clinical trials. AMRIX was studied in two double-blind, parallel-group, placebo-controlled, active-controlled trials of identical design
The most common adverse reactions (incidence ≥3% in any treatment group and greater than placebo) were dry mouth, dizziness, fatigue, constipation, nausea, dyspepsia, and somnolence (see Table 1).
Table 1: Incidence of the Most Common Adverse Reactions Occurring in ≥ 3% of Patients in any Treatment Group* and Greater Than Placebo in the Two Phase 3, Double-Blind AMRIX Trials
*AMRIX 15 mg QD, AMRIX 30 mg QD, or cyclobenzaprine IR tablets TID
5.2Postmarketing Experience
The following adverse reactions have been reported in clinical studies or postmarketing experience with AMRIX, cyclobenzaprine IR, or tricyclic drugs. Because some of these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
In a postmarketing surveillance program of cyclobenzaprine IR, the adverse reactions reported most frequently were drowsiness, dry mouth, and dizziness and adverse reactions reported in 1% to 3% of the patients were: fatigue/tiredness, asthenia, nausea, constipation, dyspepsia, unpleasant taste, blurred vision, headache, nervousness, and confusion.
The following adverse reactions have been reported in postmarketing experience (AMRIX or cyclobenzaprine IR), in clinical studies of cyclobenzaprine IR (incidence <1%), or in postmarketing experience with other tricyclic drugs:
Body as a Whole: Syncope; malaise; chest pain; edema.
Cardiovascular: Tachycardia; arrhythmia; vasodilatation; palpitation; hypotension; hypertension; myocardial infarction; heart block; stroke.
Digestive: Vomiting; anorexia; diarrhea; gastrointestinal pain; gastritis; thirst; flatulence; edema of the tongue; abnormal liver function and rare reports of hepatitis, jaundice, and cholestasis; paralytic ileus, tongue discoloration; stomatitis; parotid swelling.
Endocrine: Inappropriate ADH syndrome.
Hematologic and Lymphatic: Purpura; bone marrow depression; leukopenia; eosinophilia; thrombocytopenia.
Hypersensitivity: Anaphylaxis; angioedema; pruritus; facial edema; urticaria; rash.
Metabolic, Nutritional, and Immune: Elevation and lowering of blood sugar levels; weight gain or loss.
Musculoskeletal: Local weakness; myalgia.
Nervous System and Psychiatric: Seizures; ataxia; vertigo; dysarthria; tremors; hypertonia; convulsions; muscle twitching; disorientation; insomnia; depressed mood; abnormal sensations; anxiety; agitation; psychosis; abnormal thinking and dreaming; hallucinations; excitement; paresthesia; diplopia; serotonin syndrome; neuroleptic malignant syndrome; decreased or increased libido; abnormal gait; delusions; aggressive behavior; paranoia; peripheral neuropathy; Bell’s palsy; alteration in EEG patterns; extrapyramidal symptoms.
Respiratory: Dyspnea.
Skin: Sweating; photosensitization; alopecia.
Special Senses: Ageusia; tinnitus.
Urogenital: Urinary frequency and/or retention; impaired urination; dilatation of urinary tract; impotence; testicular swelling; gynecomastia; breast enlargement; galactorrhea.
6DRUG INTERACTIONS
Based on its structural similarity to tricyclic antidepressants, AMRIX may have life-threatening interactions with MAO inhibitors
Postmarketing cases of serotonin syndrome have been reported during combined use of cyclobenzaprine and other drugs, such as SSRIs, SNRIs, TCAs, tramadol, bupropion, meperidine, verapamil, or MAO inhibitors
7OVERDOSAGE
Clinical Presentation
Although rare, deaths may occur from overdosage with AMRIX. Multiple drug ingestion (including alcohol) is common in deliberate cyclobenzaprine overdose.
The most common effects associated with cyclobenzaprine overdose are drowsiness and tachycardia. Less frequent symptoms include tremor, agitation, coma, ataxia, hypertension, slurred speech, confusion, dizziness, nausea, vomiting, and hallucinations. Rare but potentially critical symptoms of overdose are cardiac arrest, chest pain, cardiac dysrhythmias, severe hypotension, seizures, cases of neuroleptic malignant syndrome and rhabdomyolysis have been reported. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of cyclobenzaprine toxicity. Other potential effects of overdosage include any of the symptoms listed under
Treatment of Overdose
General
As management of overdose is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment.
In order to protect against the rare but potentially critical symptoms described above, obtain an ECG and immediately initiate cardiac monitoring. Protect the patient’s airway, establish an intravenous line, and initiate gastric decontamination. Observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is necessary. If signs of toxicity occur at any time during this period, extended monitoring is required. Monitoring of plasma drug levels should not guide management of the patient. Dialysis is probably of no value because of low plasma concentrations of the drug.
Gastrointestinal Decontamination
All patients suspected of an overdose with AMRIX should receive gastrointestinal decontamination. This should include large volume gastric lavage followed by activated charcoal. If consciousness is impaired, the airway should be secured prior to lavage and emesis is contraindicated.
Cardiovascular
A maximal limb-lead QRS duration of 0.10 seconds may be the best indication of the severity of the overdose. Serum alkalinization, to a pH of 7.45 to 7.55, using intravenous sodium bicarbonate and hyperventilation (as needed), should be instituted for patients with dysrhythmias and/or QRS widening. A pH >7.60 or a pCO
CNS
In patients with CNS depression, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines or, if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin). Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in close consultation with a poison control center.
Psychiatric Follow-Up
Since overdosage is often deliberate, patients may attempt suicide by other means during the recovery phase. Psychiatric referral may be appropriate.
Pediatric Management
The principles of management of child and adult overdosage are similar. It is strongly recommended that the physician contact the local poison control center for specific pediatric treatment.
8DESCRIPTION
AMRIX is a skeletal muscle relaxant which relieves muscle spasm of local origin without interfering with muscle function. The active ingredient in AMRIX extended-release capsules is cyclobenzaprine hydrochloride, USP. Cyclobenzaprine hydrochloride (HCl) is a white, crystalline tricyclic amine salt with the empirical formula C
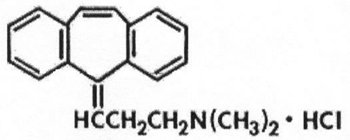
AMRIX extended-release capsules for oral administration are supplied in 15 and 30 mg strengths. AMRIX capsules contain the following inactive ingredients: diethyl phthalate NF, ethylcellulose NF (Ethocel Standard 10 Premium), gelatin, Opadry
9CLINICAL STUDIES
Efficacy was assessed in two double-blind, parallel-group, active-controlled, placebo-controlled studies of identical design of AMRIX 15 mg and 30 mg taken once daily, between 6:00 and 7:00 PM, cyclobenzaprine 10 mg three times a day, or placebo for 14 days in patients with muscle spasms associated with acute painful musculoskeletal conditions.
There were significant differences in the primary efficacy analysis, the patient’s rating of medication helpfulness, between the AMRIX 15 mg group and the placebo group at Days 4 and 14 in one study and between the AMRIX 30 mg group and the placebo group at Day 4 in the second study.
Table 2: Patients’ Rating of Medication Helpfulness - Study 1*
* Percentages are rounded to the nearest whole percent.
Table 3: Patients’ Rating of Medication Helpfulness - Study 2*
* Percentages are rounded to the nearest whole percent.
In addition, one of the two studies demonstrated significant differences between the AMRIX 30 mg group and the placebo group in terms of patient-rated relief from local pain due to muscle spasm at Day 4 and Day 8, in patient-rated restriction of movement at Day 4 and Day 8, and in patient-rated global impression of change at Day 4, Day 8, and Day 14.
In both studies, there were no significant treatment differences between the AMRIX treatment groups and the placebo group in physician's global assessment, patient-rated restriction in activities of daily living, or quality of nighttime sleep.
10PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
- Instruct patients to swallow AMRIX capsules intact or to sprinkle capsule contents on a tablespoon of applesauce and swallow immediately without chewing.
- Advise patients to stop taking AMRIX and to notify their physician right away if they experience symptoms of an allergic reaction, such as difficulty breathing, hives, swelling of face or tongue, or itching.
- Advise patients that AMRIX should not be taken with MAO inhibitors or within 14 days after their discontinuation.
- Caution patients about the risk of serotonin syndrome with concomitant use of AMRIX and other drugs, such as SSRIs, SNRIs, TCAs, tramadol, bupropion, meperidine, verapamil, or MAO inhibitors. Advise patients of the signs and symptoms of serotonin syndrome
- Advise patients to stop taking AMRIX and to notify their physician right away if they experience arrhythmias or tachycardia.
- Advise patients that AMRIX may enhance the impairment effects of alcohol. These effects may also be seen if AMRIX is taken with other CNS depressants.
- Caution patients about operating an automobile or other hazardous machinery until it is reasonably certain that AMRIX therapy will not adversely affect their ability to engage in such activities.
- Advise patients to take AMRIX at approximately the same time each day.
Manufactured for:
Manufactured By:
AMR-012
©2024 Teva Pharmaceuticals USA, Inc.
All rights reserved.
11Patient Information
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: April 2024
12INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
AMRIX
(cyclobenzaprine hydrochloride extended-release capsules)
Read this Instructions for Use before you prepare your first dose of AMRIX mixed with applesauce using the capsule sprinkle method, each time you get a refill, and as needed. There may be new information. Ask your healthcare provider or pharmacist if you have any questions about how to mix or give a dose of AMRIX using the capsule sprinkle method.
Important Information:
- Do not chew AMRIX capsules or the granules that are in the capsules.
- The capsule sprinkle method for mixing the contents of AMRIX with applesauce may be used for adults who cannot swallow capsules.
Preparing a dose of AMRIX using the capsule sprinkle method.
Before you prepare a dose of AMRIX mixed with applesauce using the capsule sprinkle method, gather the following supplies:
- paper towels
- tablespoon
- applesauce
- cup of water
How should I store AMRIX?
- Store AMRIX capsules at room temperature between 68°F to 77°F (20°C to 25°C).
Keep AMRIX capsules and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
AMRIFU-001
Issued: April 2019
13Package/Label Display Panel
NDC 63459-700-60
60 Capsules
60 Capsules
amrix(Cyclobenzaprine Hydrochloride Extended-Release Capsules)
15 mg
Rx ONLY
teva

14Package/Label Display Panel
NDC 63459-701-60
60 Capsules
60 Capsules
amrix® (Cyclobenzaprine Hydrochloride Extended-Release Capsules)
30 mg
Rx ONLY
teva
