Generic Name
Enalapril
Brand Names
Vasotec, Epaned, Vaseretic
FDA approval date: December 24, 1985
Classification: Angiotensin Converting Enzyme Inhibitor
Form: Tablet, Solution
What is Vasotec (Enalapril)?
Hypertension Enalapril maleate tablets USP are indicated for the treatment of hypertension. Enalapril maleate tablets USP are effective alone or in combination with other antihypertensive agents, especially thiazide- type diuretics. The blood pressure lowering effects of enalapril maleate tablets USP and thiazides are approximately additive. Heart Failure Enalapril maleate tablets USP are indicated for the treatment of symptomatic congestive heart failure, usually in combination with diuretics and digitalis. In these patients enalapril maleate tablets USP improves symptoms, increases survival, and decreases the frequency of hospitalization. Asymptomatic Left Ventricular Dysfunction In clinically stable asymptomatic patients with left ventricular dysfunction , enalapril maleate tablets USP decreases the rate of development of overt heart failure and decreases the incidence of hospitalization for heart failure. In using enalapril maleate tablets USP consideration should be given to the fact that another angiotensin converting enzyme inhibitor, captopril, has caused agranulocytosis, particularly in patients with renal impairment or collagen vascular disease, and that available data are insufficient to show that enalapril maleate tablets USP does not have a similar risk. In considering use of enalapril maleate tablets USP, it should be noted that in controlled clinical trials ACE inhibitors have an effect on blood pressure that is less in black patients than in non-blacks. In addition, it should be noted that black patients receiving ACE inhibitors have been reported to have a higher incidence of angioedema compared to non-blacks.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Vasotec (Enalapril Maleate)
WARNING: FETAL TOXICITY
See full prescribing information for complete boxed warning.
- When pregnancy is detected, discontinue VASOTEC
- Drugs that act directly on the renin-angiotensin system can cause injury and death to thedeveloping fetus. (SeeWARNINGS, Fetal Toxicity.)
1DESCRIPTION
VASOTEC
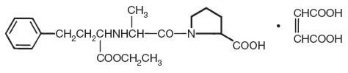
Enalapril maleate is a white to off-white, crystalline powder with a molecular weight of 492.53. It is sparingly soluble in water, soluble in ethanol, and freely soluble in methanol.
Enalapril is a pro-drug; following oral administration, it is bioactivated by hydrolysis of the ethyl ester to enalaprilat, which is the active angiotensin-converting enzyme inhibitor.
Enalapril maleate is supplied as 2.5 mg, 5 mg, 10 mg, and 20 mg tablets for oral administration. In addition to the active ingredient enalapril maleate, each tablet contains the following inactive ingredients: lactose, magnesium stearate, sodium bicarbonate, and starch. The 10 mg and 20 mg tablets also contain iron oxides.
2CONTRAINDICATIONS
VASOTEC is contraindicated in patients who are hypersensitive to this product and in patients with a history of angioedema related to previous treatment with an angiotensin-converting enzyme inhibitor and in patients with hereditary or idiopathic angioedema.
Do not coadminister aliskiren with VASOTEC in patients with diabetes (see
VASOTEC is contraindicated in combination with a neprilysin inhibitor (e.g., sacubitril). Do not administer VASOTEC within 36 hours of switching to or from sacubitril/valsartan, a neprilysin inhibitor (see
3ADVERSE REACTIONS
VASOTEC has been evaluated for safety in more than 10,000 patients, including over 1000 patients treated for one year or more. VASOTEC has been found to be generally well tolerated in controlled clinical trials involving 2987 patients. For the most part, adverse experiences were mild and transient in nature. In clinical trials, discontinuation of therapy due to clinical adverse experiences was required in 3.3 percent of patients with hypertension and in 5.7 percent of patients with heart failure. The frequency of adverse experiences was not related to total daily dosage within the usual dosage ranges. In patients with hypertension the overall percentage of patients treated with VASOTEC reporting adverse experiences was comparable to placebo.
4OVERDOSAGE
Limited data are available in regard to overdosage in humans.
Single oral doses of enalapril above 1,000 mg/kg and ≥1,775 mg/kg were associated with lethality in mice and rats, respectively.
The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of normal saline solution.
Enalaprilat may be removed from general circulation by hemodialysis and has been removed from neonatal circulation by peritoneal dialysis (see
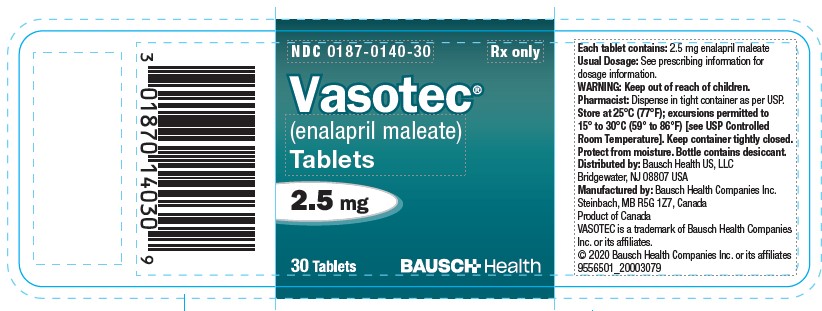
5PRINCIPAL DISPLAY PANEL - 2.5 mg Bottle Label
NDC 0187-0140-30
Rx Only
Vasotec®
(enalapril maleate)
Tablets
(enalapril maleate)
Tablets
2.5 mg
30 Tablets
BAUSCH Health
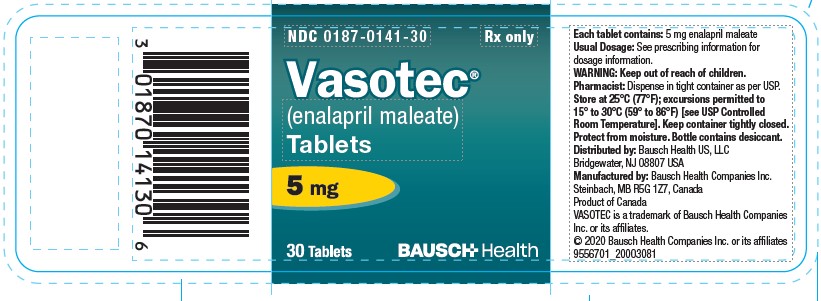
6PRINCIPAL DISPLAY PANEL - 5 mg Bottle Label
NDC 0187-0141-30
Rx only
Vasotec®
(enalapril maleate)
Tablets
(enalapril maleate)
Tablets
5 mg
30 Tablets
BAUSCH Health
7PRINCIPAL DISPLAY PANEL - 10 mg Bottle Label
NDC 0187-0142-30
Vasotec®
(enalapril maleate)
Tablets
(enalapril maleate)
Tablets
10 mg
30 Tablets
BAUSCH Health

8PRINCIPAL DISPLAY PANEL - 20 mg Bottle Label
NDC 0187-0143-30
Rx only
Vasotec®
(enalapril maleate)
Tablets
(enalapril maleate)
Tablets
20 mg
30 Tablets
BAUSCH Health
