Brand Name
Inspra
Generic Name
Eplerenone
View Brand Information FDA approval date: September 27, 2002
Classification: Aldosterone Antagonist
Form: Tablet
What is Inspra (Eplerenone)?
Eplerenone tablets is an aldosterone antagonist indicated for: Improving survival of stable patients with symptomatic heart failure with reduced ejection fraction after an acute myocardial infarction.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Inspra (eplerenone)
1DOSAGE FORMS AND STRENGTHS
- 25 mg tablets: yellow diamond biconvex film-coated tablets debossed with “VLE” on one side and “NSR” over “25” on the other
- 50 mg tablets: yellow diamond biconvex film-coated tablets debossed with “VLE” on one side and “NSR” over “50” on the other
2CONTRAINDICATIONS
For All Patients
INSPRA is contraindicated in all patients with:
- serum potassium >5.5 mEq/L at initiation,
- creatinine clearance ≤30 mL/min, or
- concomitant administration of strong CYP3A inhibitors (e.g., ketoconazole, itraconazole, nefazodone, troleandomycin, clarithromycin, ritonavir, and nelfinavir)
For Patients Treated for Hypertension
INSPRA is contraindicated for the treatment of hypertension in patients with:
- type 2 diabetes with microalbuminuria,
- serum creatinine >2.0 mg/dL in males or >1.8 mg/dL in females,
- creatinine clearance <50 mL/min, or
- concomitant administration of potassium supplements or potassium-sparing diuretics (e.g., amiloride, spironolactone, or triamterene)
3ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Hyperkalemia
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
3.1.1Heart Failure Post-Myocardial Infarction
In EPHESUS, safety was evaluated in 3307 patients treated with INSPRA and 3301 placebo-treated patients. The overall incidence of adverse events reported with INSPRA (78.9%) was similar to placebo (79.5%). Adverse events occurred at a similar rate regardless of age, gender, or race. Patients discontinued treatment due to an adverse event at similar rates in either treatment group (4.4% INSPRA vs. 4.3% placebo), with the most common reasons for discontinuation being hyperkalemia, MI, and abnormal renal function.
Adverse reactions that occurred more frequently in patients treated with INSPRA than placebo were hyperkalemia (3.4% vs. 2.0%) and increased creatinine (2.4% vs. 1.5%). Discontinuations due to hyperkalemia or abnormal renal function were less than 1.0% in both groups.
3.1.2Hypertension
INSPRA has been evaluated for safety in 3091 patients treated for hypertension. A total of 690 patients were treated for over 6 months and 106 patients were treated for over 1 year.
In placebo-controlled studies, the overall rates of adverse events were 47% with INSPRA and 45% with placebo. Adverse events occurred at a similar rate regardless of age, gender, or race. Therapy was discontinued due to an adverse event in 3% of patients treated with INSPRA and 3% of patients given placebo. The most common reasons for discontinuation of INSPRA were headache, dizziness, angina pectoris/MI, and increased GGT.
Gynecomastia and abnormal vaginal bleeding were reported with INSPRA but not with placebo. The rates increased with increasing duration of therapy.
3.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of INSPRA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin: angioedema, rash
4OVERDOSAGE
No cases of human overdosage with eplerenone have been reported. Lethality was not observed in mice, rats, or dogs after single oral doses that provided C
The most likely manifestation of human overdosage would be anticipated to be hypotension or hyperkalemia. Eplerenone cannot be removed by hemodialysis. Eplerenone has been shown to bind extensively to charcoal. If symptomatic hypotension should occur, supportive treatment should be instituted. If hyperkalemia develops, standard treatment should be initiated.
5DESCRIPTION
INSPRA contains eplerenone, a blocker of aldosterone binding at the mineralocorticoid receptor.
Eplerenone is chemically described as Pregn-4-ene-7,21-dicarboxylic acid, 9,11-epoxy-17-hydroxy-3-oxo-, γ-lactone, methyl ester, (7α,11α,17α)-. Its empirical formula is C
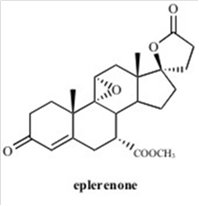
Eplerenone is an odorless, white to off-white crystalline powder. It is very slightly soluble in water, with its solubility essentially pH-independent. The octanol/water partition coefficient of eplerenone is approximately 7.1 at pH 7.0.
INSPRA tablets for oral administration contain 25 mg or 50 mg of eplerenone and the following inactive ingredients: croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, red iron oxide, sodium lauryl sulfate, talc, titanium dioxide, and yellow iron oxide.
6HOW SUPPLIED/STORAGE AND HANDLING
INSPRA Tablets are yellow, diamond biconvex, and film-coated. They are debossed with “VLE” on one side. They are supplied as follows:
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [See USP Controlled Room Temperature].
7PATIENT COUNSELING INFORMATION
Advise patients receiving INSPRA:
- Not to use potassium supplements or salt substitutes containing potassium without consulting the prescribing physician
- To call their physician if they experience dizziness, diarrhea, vomiting, rapid or irregular heartbeat, lower extremity edema, or difficulty breathing
Distributed by:
© 2025 Viatris Inc.
INSPRA is a registered trademark of UPJOHN US 2 LLC, a Viatris Company.
UPJ:INSPRA:R2
8PRINCIPAL DISPLAY PANEL – 25 mg
NDC 58151-142-93
INSPRA
25 mg
30 Tablets
Rx only
Store at 25°C (77°F);
[see USP Controlled
Room Temperature].
[see USP Controlled
Room Temperature].
Dispense in tight (USP),
DOSAGE AND USE
See accompanying
prescribing information.
Each tablet contains
25 mg eplerenone.
See accompanying
prescribing information.
Each tablet contains
25 mg eplerenone.
Distributed by:
© 2023 Viatris Inc.
RUPJ142H
9PRINCIPAL DISPLAY PANEL – 50 mg
NDC 58151-143-93
INSPRA
50 mg
30 Tablets
Rx only
Store at 25°C (77°F);
[see USP Controlled
Room Temperature].
[see USP Controlled
Room Temperature].
Dispense in tight (USP),
DOSAGE AND USE
See accompanying
prescribing information.
Each tablet contains
50 mg eplerenone.
See accompanying
prescribing information.
Each tablet contains
50 mg eplerenone.
Distributed by:
© 2023 Viatris Inc.
RUPJ143H
