Brand Name
Ebglyss
Generic Name
Lebrikizumab-Lbkz
View Brand Information FDA approval date: September 13, 2024
Classification: Interleukin-13 Antagonist
Form: Injection
What is Ebglyss (Lebrikizumab-Lbkz)?
EBGLYSS is indicated for the treatment of adults and pediatric patients 12 years of age and older who weigh at least 40 kg with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. EBGLYSS can be used with or without topical corticosteroids. EBGLYSS™ is an interleukin-13 antagonist indicated for the treatment of adults and pediatric patients 12 years of age and older who weigh at least 40 kg with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. EBGLYSS can be used with or without topical corticosteroids.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
EBGLYSS (lebrikizumab-lbkz)
1INDICATIONS AND USAGE
EBGLYSS is indicated for the treatment of adults and pediatric patients 12 years of age and older who weigh at least 40 kg with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. EBGLYSS can be used with or without topical corticosteroids.
2DOSAGE FORMS AND STRENGTHS
EBGLYSS is a clear to opalescent, colorless to slightly yellow to slightly brown solution available as follows:
- Injection: 250 mg/2 mL in a single-dose prefilled pen
- Injection: 250 mg/2 mL (125 mg/mL) in a single-dose prefilled syringe with needle shield
3CONTRAINDICATIONS
EBGLYSS is contraindicated in patients with prior serious hypersensitivity to lebrikizumab-lbkz or any excipients of EBGLYSS
4ADVERSE REACTIONS
The following adverse reactions are described elsewhere in the labeling:
- Hypersensitivity
- Conjunctivitis and Keratitis
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying and controlled conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
5OVERDOSAGE
In the event of overdosage, contact Poison Control (1-800-222-1222) for the latest recommendations and monitor the patient for any signs or symptoms of adverse reactions and institute appropriate symptomatic treatment immediately.
6DESCRIPTION
Lebrikizumab-lbkz, an interleukin-13 antagonist, is an immunoglobulin G4 (IgG4) monoclonal antibody that binds to interleukin (IL)-13 and inhibits IL-13 signaling. Lebrikizumab-lbkz is produced in Chinese Hamster Ovary (CHO) cells by recombinant DNA technology. Lebrikizumab-lbkz has an approximate molecular weight of 145 kDa.
EBGLYSS (lebrikizumab-lbkz) injection is a sterile, preservative free, clear to opalescent, colorless to slightly yellow to slightly brown solution for subcutaneous use. EBGLYSS is available as either a 250 mg/2 mL single-dose prefilled pen or a single-dose prefilled syringe with needle shield. The EBGLYSS prefilled pen and prefilled syringe with needle shield are not made with natural rubber latex.
Each prefilled pen or prefilled syringe delivers 250 mg lebrikizumab-lbkz in 2 mL solution which also contains glacial acetic acid (1.8 mg), histidine (6.2 mg), polysorbate 20 (0.6 mg), sucrose (119.6 mg) and Water for Injection. The pH is 5.4 – 6.0.
7PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
8PREFILLED PEN INSTRUCTIONS FOR USE
Read the Patient Information insert for EBGLYSS inside this box to learn more about your medicine.
Eli Lilly and Company
EBGLYSS is a trademark of Eli Lilly and Company.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
9PREFILLED SYRINGE INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
EBGLYSS™[EHB-glihs]
(lebrikizumab-lbkz)
injection, for subcutaneous use
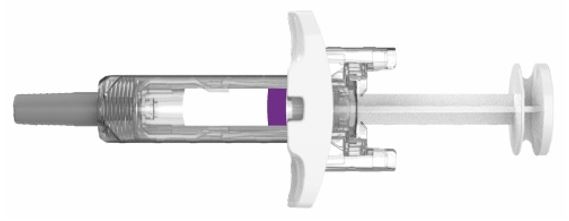
Single-Dose Prefilled Syringe with Needle Shield
This Instructions for Use contains information on how to inject EBGLYSS.
Before you use the EBGLYSS Prefilled Syringe with Needle Shield (Prefilled Syringe), read and carefully follow all the step-by-step instructions.
Important information you need to know before injecting EBGLYSS
- Your healthcare provider should show you how to prepare and inject EBGLYSS using the Prefilled Syringe.
- Keep these Instructions for Use and read it as needed.
- Each EBGLYSS Prefilled Syringe contains 1 dose of EBGLYSS.
- The EBGLYSS Prefilled Syringe contains glass parts. Handle it carefully. If you drop it on a hard surface,
- Your healthcare provider may help you decide where on your body to inject your dose. You can also read the
- If you have vision problems,
- See
INSTRUCTIONS FOR USE
Before you use the EBGLYSS Prefilled Syringe, read and carefully follow all the step-by-step instructions.
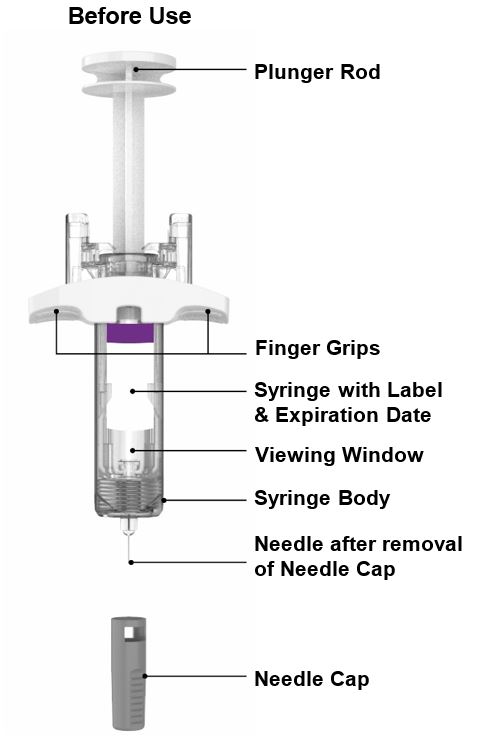
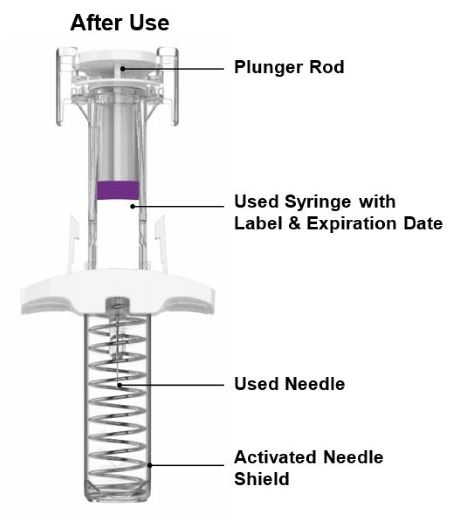
Parts of the EBGLYSS Prefilled Syringe with Needle Shield
Storing EBGLYSS
- Store your Prefilled Syringe in a refrigerator between 36°F to 46°F (2°C to 8°C).
- EBGLYSS can be stored at room temperature up to 7 days in the original carton.
- Store your Prefilled Syringe in the original carton to protect from light until use.
- Do not freeze your Prefilled Syringe. Do not shake your Prefilled Syringe.
- Do not microwave your Prefilled Syringe, or run hot water over it, or leave it in direct sunlight.
- Throw away (dispose of) your Prefilled Syringe if any of the above conditions are not followed.
Keep your Prefilled Syringes and all medicines out of the reach of children.
Read the Patient Information insert for EBGLYSS inside this box to learn more about your medicine.
Eli Lilly and Company
Indianapolis, IN 46285, USA
US License Number 1891
EBGLYSS is a trademark of Eli Lilly and Company.
Copyright © 2024, 2025, Eli Lilly and Company. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: October 2025
EBG-0002-PFS-IFU-20251028


