Brand Name
Bepreve
Generic Name
Bepotastine Besilate
View Brand Information FDA approval date: September 08, 2009
Classification: Histamine-1 Receptor Antagonist
Form: Solution
What is Bepreve (Bepotastine Besilate)?
Bepotastine Besilate Ophthalmic Solution, 1.5% is a histamine H 1 receptor antagonist indicated for the treatment of itching associated with signs and symptoms of allergic conjunctivitis. Bepotastine Besilate Ophthalmic Solution is a histamine H 1 receptor antagonist indicated for the treatment of itching associated with allergic conjunctivitis.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Bepreve (bepotastine besilate)
1INDICATIONS AND USAGE
BEPREVE
2DOSAGE AND ADMINISTRATION
Instill one drop of BEPREVE into the affected eye(s) twice a day. Remove contact lenses prior to instillation of BEPREVE.
3DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing bepotastine besilate 15 mg/mL (1.5%).
4CONTRAINDICATIONS
BEPREVE is contraindicated in patients with a history of hypersensitivity reactions to bepotastine or any of the other ingredients
5DESCRIPTION
BEPREVE (bepotastine besilate ophthalmic solution) 1.5% is a sterile, topically administered drug for ophthalmic use. Each mL of BEPREVE contains 15 mg bepotastine besilate. Bepotastine besilate is designated chemically as (+) -4-[[(S)-p-chloro-alpha -2-pyridylbenzyl]oxy]-1-piperidine butyric acid monobenzenesulfonate. The chemical structure for bepotastine besilate is:

Bepotastine besilate is a white to pale yellowish-white crystalline powder. The molecular weight of bepotastine besilate is 547.06 daltons. BEPREVE ophthalmic solution is supplied as a sterile, aqueous 1.5% solution, with an approximate pH of 6.8. The osmolality of BEPREVE (bepotastine besilate ophthalmic solution) 1.5% is approximately 295 mOsm/kg.
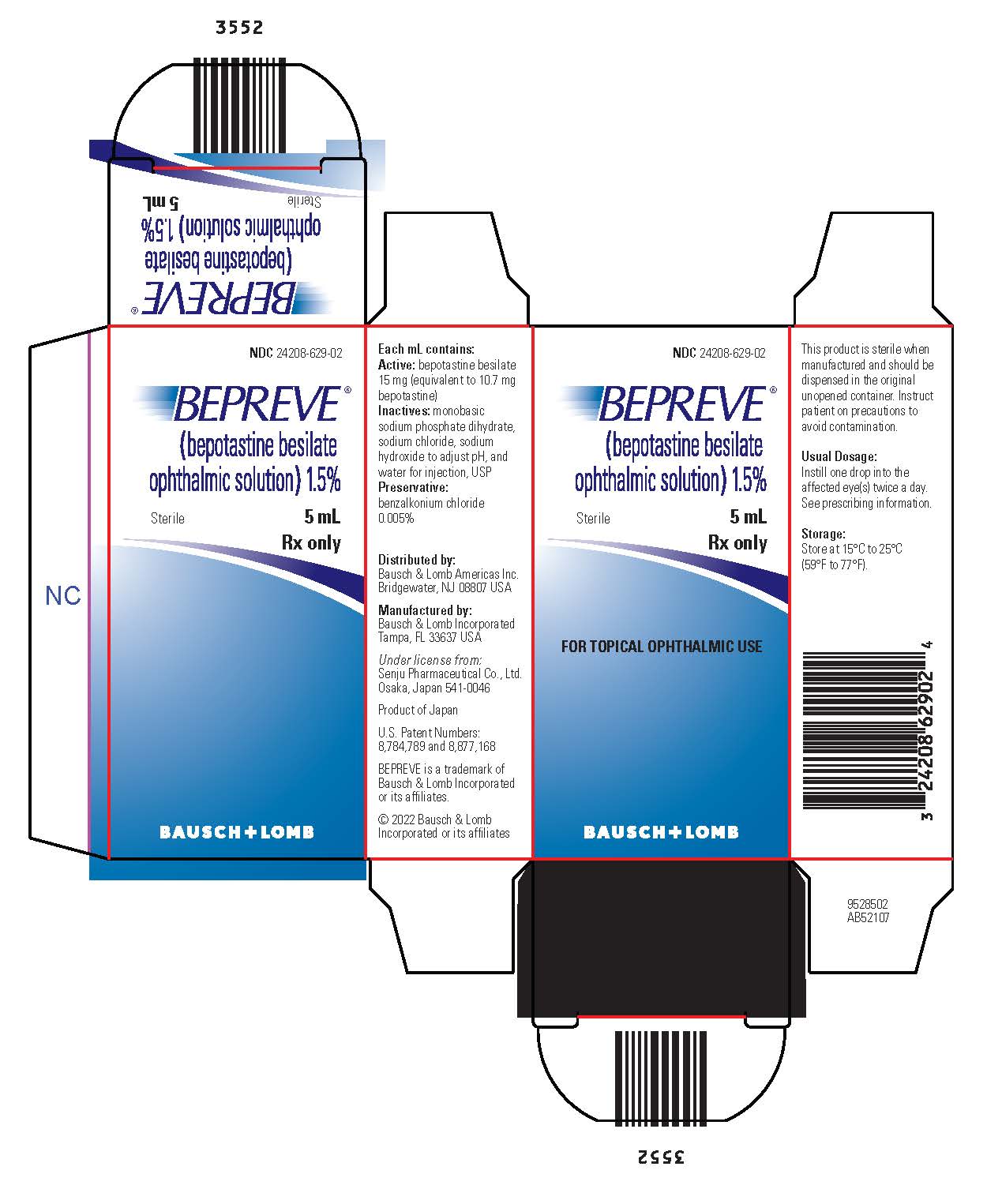
Each mL of BEPREVE (bepotastine besilate ophthalmic solution) 1.5% contains:
- Active: bepotastine besilate 15 mg (equivalent to 10.7 mg bepotastine)
- Inactives: monobasic sodium phosphate dihydrate, sodium chloride, sodium hydroxide to adjust pH, and water for injection, USP
- Preservative: benzalkonium chloride 0.005%
6CLINICAL STUDIES
Clinical efficacy was evaluated in two conjunctival allergen challenge (CAC) studies (237 patients). BEPREVE (bepotastine besilate ophthalmic solution) 1.5% was more effective than its vehicle for relieving ocular itching induced by an ocular allergen challenge, both at a CAC 15 minutes post-dosing and a CAC 8 hours post-dosing of BEPREVE.
The safety of BEPREVE was evaluated in a randomized clinical study of 861 subjects over a period of 6 weeks.
7HOW SUPPLIED/STORAGE AND HANDLING
BEPREVE (bepotastine besilate ophthalmic solution) 1.5% is supplied in a white low density polyethylene bottle with a sterile linear low density polyethylene controlled dropper tip and a white polypropylene cap in the following sizes:
NDC 24208-629-02 5 mL Bottle
Storage:
Store at 15°C to 25°C (59°F to 77°F).
8PATIENT COUNSELING INFORMATION
- Sterility of Dropper Tip
- Advise patients not to touch the dropper tip to any surface, as this may contaminate the solution and to keep the bottle tightly closed when not in use.
Distributed by:
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch & Lomb Incorporated
Tampa, FL 33637 USA
Under license from:
Senju Pharmaceutical Co., Ltd.
Osaka, Japan 541-0046
U.S. Patent Numbers: 8,784,789 and 8,877,168
Bausch & Lomb Incorporated
Tampa, FL 33637 USA
Under license from:
Senju Pharmaceutical Co., Ltd.
Osaka, Japan 541-0046
U.S. Patent Numbers: 8,784,789 and 8,877,168
BEPREVE is a trademark of Bausch & Lomb Incorporated or its affiliates.
© 2022 Bausch & Lomb Incorporated or its affiliates
9291104 (folded)
9PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 24208-629-02
BEPREVE
(bepotastine besilate
Sterile
Rx only
FOR TOPICAL OPHTHALMIC USE
FOR TOPICAL OPHTHALMIC USE
BAUSCH + LOMB
9528502