Brand Name
Cancidas
Generic Name
Caspofungin Acetate
View Brand Information FDA approval date: January 26, 2001
Classification: Echinocandin Antifungal
Form: Injection
What is Cancidas (Caspofungin Acetate)?
Caspofungin acetate for Injection is an echinocandin antifungal indicated in adults and pediatric patients for: Empirical therapy for presumed fungal infections in febrile, neutropenic patients. Treatment of candidemia and the following Candida infections: intra-abdominal abscesses, peritonitis and pleural space infections. Treatment of esophageal candidiasis. Treatment of invasive aspergillosis in patients who are refractory to or intolerant of other therapies.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
CANCIDAS (CASPOFUNGIN ACETATE)
1DOSAGE FORMS AND STRENGTHS
CANCIDAS 50 mg is a white to off-white lyophilized cake or powder for reconstitution in a single-dose glass vial with a red aluminum band and a plastic cap. CANCIDAS 50-mg vial contains 50 mg of caspofungin equivalent to 55.5 mg of caspofungin acetate.
CANCIDAS 70 mg is a white to off-white lyophilized cake or powder for reconstitution in a single-dose glass vial with a yellow/orange aluminum band and a plastic cap. CANCIDAS 70-mg vial contains 70 mg of caspofungin equivalent to 77.7 mg of caspofungin acetate.
2CONTRAINDICATIONS
CANCIDAS is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to any component of this product
3ADVERSE REACTIONS
The following serious adverse reactions are discussed in detail in another section of the labeling:
- Hypersensitivity
- Hepatic Effects
- Elevated Liver Enzymes During Concomitant Use With Cyclosporine
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of CANCIDAS cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
3.2Postmarketing Experience
The following additional adverse reactions have been identified during the post-approval use of CANCIDAS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Gastrointestinal disorders: pancreatitis
- Hepatobiliary disorders: hepatic necrosis
- Skin and subcutaneous tissue disorders: erythema multiforme, toxic epidermal necrolysis, Stevens-Johnson syndrome, and skin exfoliation
- Renal and urinary disorders: clinically significant renal dysfunction
- General disorders and administration site conditions: swelling and peripheral edema
- Laboratory abnormalities: gamma-glutamyltransferase increased
4OVERDOSAGE
In 6 healthy subjects who received a single 210-mg dose, no significant adverse reactions were reported. Multiple doses above 150 mg daily have not been studied. Caspofungin is not dialyzable.
In clinical trials, one pediatric patient (16 years of age) unintentionally received a single dose of caspofungin of 113 mg (on Day 1), followed by 80 mg daily for an additional 7 days. No clinically significant adverse reactions were reported.
5DESCRIPTION
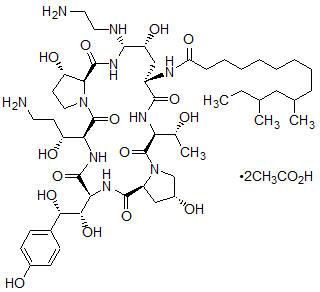
CANCIDAS is a sterile, lyophilized product for intravenous (IV) infusion that contains a semisynthetic lipopeptide (echinocandin) compound synthesized from a fermentation product of
CANCIDAS (caspofungin acetate) is 1-[(4
Caspofungin acetate is a hygroscopic, white to off-white powder. It is freely soluble in water and methanol, and slightly soluble in ethanol. The pH of a saturated aqueous solution of caspofungin acetate is approximately 6.6. The empirical formula is C
6REFERENCES
- Mosteller RD: Simplified Calculation of Body Surface Area. N Engl J Med 1987 Oct 22;317(17): 1098 (letter).
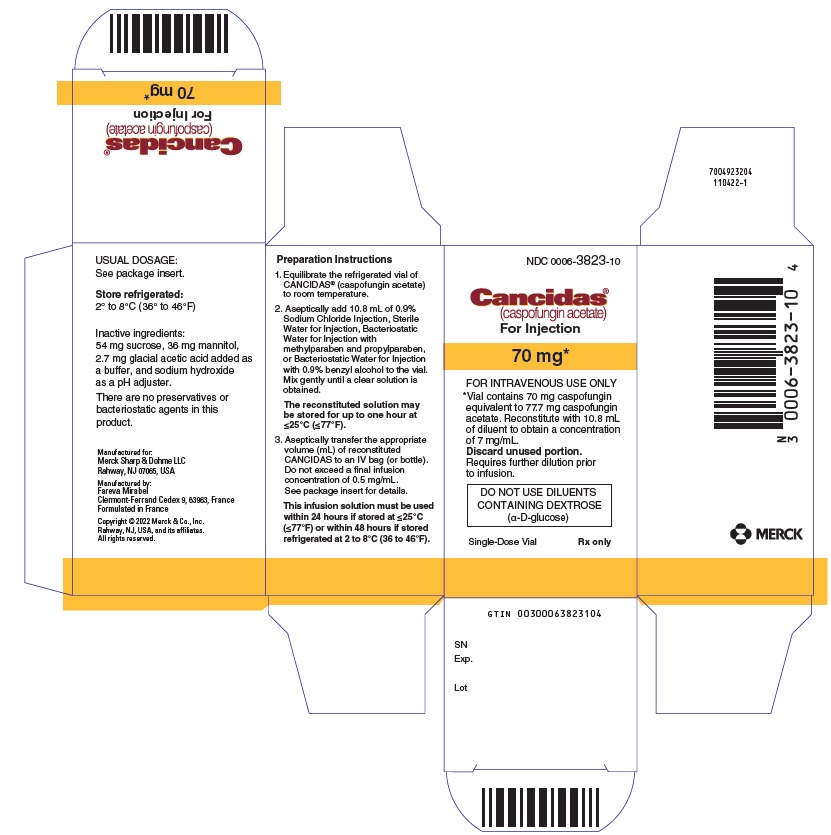
7PRINCIPAL DISPLAY PANEL - 50 mg Vial Carton
NDC 0006-3822-10
Cancidas®
(caspofungin acetate)
For Injection
(caspofungin acetate)
For Injection
50 mg*
FOR INTRAVENOUS USE ONLY
*Vial contains 50 mg caspofungin
DO NOT USE DILUENTS
Single-Dose Vial
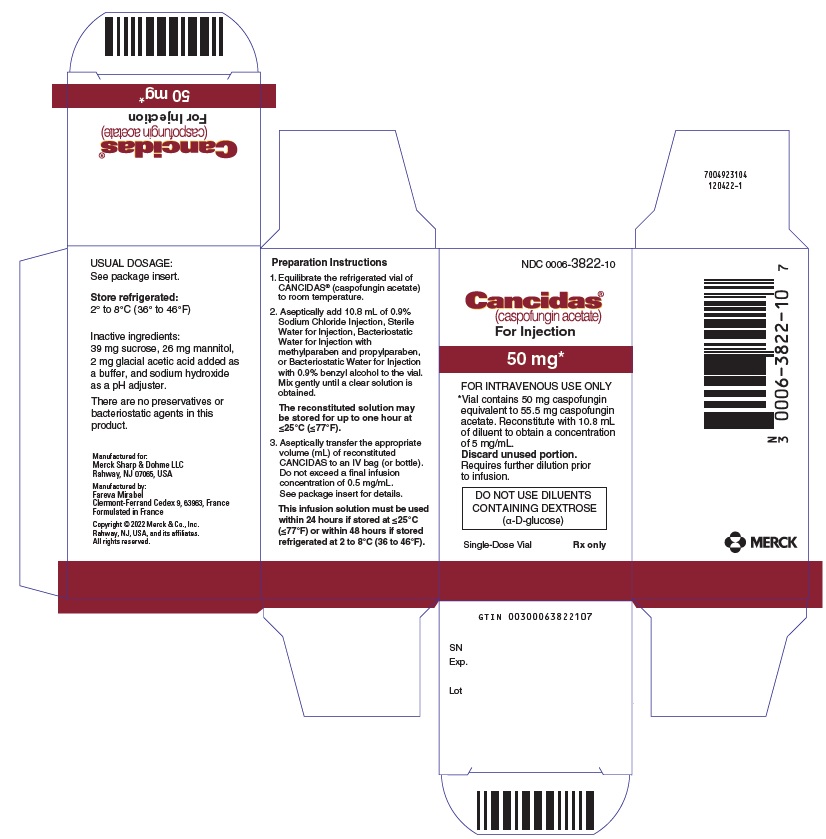
8PRINCIPAL DISPLAY PANEL - 70 mg Vial Carton
NDC 0006-3823-10
Cancidas®
(caspofungin acetate)
For Injection
(caspofungin acetate)
For Injection
70 mg*
FOR INTRAVENOUS USE ONLY
*Vial contains 70 mg caspofungin
DO NOT USE DILUENTS
Single-Dose Vial