Cypionate
What is Depo-Estradiol (Cypionate)?
As women move through menopause or experience conditions that lower estrogen levels, the changes in their bodies can feel both physical and emotional hot flashes, mood swings, and night sweats can interrupt daily life and sleep. For others, hormonal changes after surgery or certain health conditions can lead to similar symptoms. Depo-Estradiol (estradiol cypionate) is a medication designed to help restore hormonal balance and improve overall quality of life by replenishing the body’s natural estrogen.
Depo-Estradiol is a form of estrogen replacement therapy used to treat symptoms associated with menopause and estrogen deficiency. It belongs to a drug class known as estrogens, the primary female hormones responsible for regulating many bodily functions including reproductive health, bone strength, and mood stability. The medication has been available for decades and is considered a long-acting injectable form of estrogen therapy, offering consistent hormone levels over time. It is typically prescribed when oral estrogen is not preferred or when a more sustained effect is needed.
What does Depo-Estradiol do?
Depo-Estradiol helps relieve symptoms of low estrogen that often occur during menopause, after ovarian surgery, or due to certain medical conditions. These symptoms can include:
- Hot flashes and night sweats
- Vaginal dryness or irritation
- Mood swings and irritability
- Reduced bone density or early signs of osteoporosis
By restoring estrogen levels, the medication helps reduce discomfort, improve mood and sleep, and protect bone health. For many women, this means fewer interruptions in daily activities and a better sense of well-being.
In addition to menopausal symptom relief, Depo-Estradiol may also be used in hormone therapy for transgender women, as part of a medically supervised regimen, or for specific hormonal disorders in women where estrogen replacement is needed.
Clinical studies and decades of use have shown that when properly prescribed and monitored, estradiol injections are effective in managing symptoms and maintaining stable estrogen levels over extended periods (NIH, 2024).
How does Depo-Estradiol work?
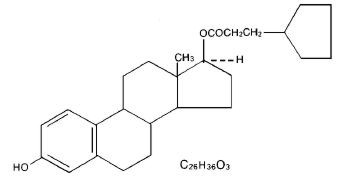
Depo-Estradiol contains estradiol cypionate, a synthetic form of the natural hormone estradiol, which is the most potent type of estrogen produced by the ovaries.
After the medication is injected into a muscle, it gradually releases estradiol into the bloodstream. This steady, controlled release mimics the body’s natural hormone patterns more closely than oral tablets, helping maintain consistent estrogen levels for several days or weeks.
Estrogen works by binding to specific receptors in tissues throughout the body including the brain, bones, skin, and reproductive organs to regulate key functions. It:
- Stabilizes body temperature control centers in the brain, reducing hot flashes and night sweats
- Promotes healthy vaginal tissue and lubrication
- Helps maintain calcium balance and bone density
- Supports mood, energy, and skin elasticity
This mechanism is clinically important because stable estrogen levels reduce symptom flare-ups and long-term complications such as osteoporosis, which can occur when estrogen levels remain low for extended periods.
Depo-Estradiol side effects
Most people tolerate Depo-Estradiol well, but like all hormone therapies, it can cause side effects. These may vary based on individual health, dosage, and duration of treatment.
Common side effects may include:
- Breast tenderness or swelling
- Headache or mild nausea
- Fluid retention or bloating
- Mood changes
- Injection site soreness
Less common but serious side effects include:
- Blood clots (deep vein thrombosis or pulmonary embolism)
- Stroke or heart attack
- Liver problems (yellowing of the skin or eyes, dark urine)
- Unusual vaginal bleeding
- Increased risk of certain cancers with long-term, unsupervised use
Depo-Estradiol is contraindicated in individuals with a history of blood clots, stroke, certain cancers (breast/uterine), or liver disease, unless a doctor advises otherwise, due to its effects on blood clotting and metabolism.
Seek immediate medical attention for chest pain, shortness of breath, leg swelling, or sudden vision changes.
Regular medical follow-ups, including pelvic exams, breast screenings, and blood pressure checks, are crucial for safe estrogen therapy. When properly monitored, Depo-Estradiol is a safe and effective treatment for most women.
Depo-Estradiol dosage
Depo-Estradiol, an intramuscular injection of estradiol cypionate, is administered by a healthcare professional every few weeks for steady hormone replacement. Dosing is individualized and adjusted by doctors to balance symptom relief with safety.
Ongoing monitoring may include blood tests for hormone levels, liver function tests for long-term users, blood pressure and cholesterol checks, and mammograms and pelvic exams. For older adults or those with cardiovascular or liver conditions, the lowest effective dose may be used to minimize risks.
Does Depo-Estradiol have a generic version?
Yes. Depo-Estradiol (estradiol cypionate) is available in brand-name and generic formulations in the United States. The generic version contains the same active ingredient, strength, and safety profile as the brand-name product and is approved by the FDA.
Pharmacies typically dispense generic forms of medication, such as Depo-Estradiol, due to their equal effectiveness and affordability, unless otherwise specified by the doctor. Depo-Estradiol should not be interchanged with other estrogen therapies like estradiol valerate (Delestrogen) or oral estradiol tablets, as they have different absorption rates and dosing intervals. Consult your healthcare provider to determine the best formulation for your medical history and treatment goals.
Conclusion
Depo-Estradiol (estradiol cypionate) is a trusted and effective option for estrogen replacement therapy, offering long-lasting relief from symptoms of menopause and other estrogen-deficiency conditions. By maintaining stable hormone levels, it helps improve comfort, bone health, and emotional well-being.
Under medical supervision, Depo-Estradiol helps patients feel more balanced, energetic, and confident. Regular checkups and adherence to dosing are crucial for safe and successful treatment. Appropriately prescribed and monitored, it restores hormonal harmony and improves quality of life for those needing estrogen replacement.
References
- U.S. Food and Drug Administration (FDA). (2024). Depo-Estradiol (estradiol cypionate) prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Estradiol injection (intramuscular route) drug information. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Estradiol injection: Uses, dosage, and side effects. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Estrogen therapy and menopausal symptom management. Retrieved from https://www.nih.gov
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- Moderate to severe
- Hypoestrogenism due to hypogonadism.
- Undiagnosed abnormal genital bleeding.
- Known or suspected cancer of the breast.
- Known or suspected estrogen-dependent neoplasia.
- Active deep vein thrombosis, pulmonary embolism or history of these conditions.
- Active or recent (e.g., within the past year) arterial thromboembolic disease (e.g., stroke, myocardial infarction).
- Liver dysfunction or disease.
- DEPO-Estradiol should not be used in patients with known hypersensitivity to its ingredients.
- Known or suspected pregnancy. There is no indication for DEPO-Estradiol in pregnancy.
- Genitourinary system
Changes in vaginal bleeding pattern and abnormal withdrawal bleeding or flow; breakthrough bleeding, spotting; dysmenorrhea; increase in size of uterine leiomyomata; vaginitis including vaginal candidiasis; change in amount of cervical secretion; changes in cervical ectropion; ovarian cancer; endometrial hyperplasia; endometrial cancer. - Breasts
Tenderness, enlargement pain, nipple discharge, galactorrhea; fibrocystic breast changes; breast cancer. - Cardiovascular
Deep and superficial venous thrombosis; pulmonary embolism; thrombophlebitis; myocardial infarction; stroke; increase in blood pressure. - Gastrointestinal
Nausea, vomiting; abdominal cramps, bloating; cholestatic jaundice; increased incidence of gallbladder disease; pancreatitis, enlargement of hepatic hemangiomas. - Skin
Chloasma or melasma that may persist when drug is discontinued. Erythema multiforme; erythema nodosum; hemorrhagic eruption; loss of scalp hair; hirsutism; pruritus, rash. - Eyes
Retinal vascular thrombosis; steepening of corneal curvature; intolerance to contact lenses. - Central nervous system
Headache, migraine, dizziness; mental depression; chorea; nervousness; mood disturbances; irritability; exacerbation of epilepsy, dementia. - Miscellaneous
Increase or decrease in weight; reduced carbohydrate tolerance; aggravation of porphyria; edema; changes in libido; arthralgias; leg cramps; anaphylactoid/anaphylactic reactions including urticaria and angioedema; hypocalcemia; exacerbation of asthma; increased triglycerides.
- Short-term cyclic use for treatment of moderate to severe vasomotor symptoms, vulval and vaginal atrophy associated with the menopause, the lowest dose and regimen that will control symptoms should be chosen and medication should be discontinued as promptly as possible.
- For treatment of female hypoestrogenism due to hypogonadism 1.5 to 2 mg injected at monthly intervals.
- Ziel HK, Finkle WD: Increased risk of endometrial carcinoma among users of conjugated estrogens.
- Smith DC, Prentice R, Thompson DJ, et al: Association of exogenous estrogen and endometrial carcinoma.
- Mack TM, Pike MC, Henderson BE, et al: Estrogens and endometrial cancer in a retirement community.
- Weiss NS, Szekely DR, Austin DF: Increasing incidence of endometrial cancer in the United States.
- Herbst AL, Ulfelder H, Poskanzer DC: Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women.
- Greenwald P, Barlow JJ, Nasca PC, Burnett WS: Vaginal cancer after maternal treatment with synthetic estrogens.
- Lanier AP, Noller KL, Decker DG, Elveback LR, Kurland LT: Cancer and stilbestrol. A follow-up of 1,719 persons exposed to estrogens
- Herbst AL, Kurman RJ, Scully RE: Vaginal and cervical abnormalities after exposure to stilbestrol
- Herbst AL, Robboy SJ, Macdonald GJ, Scully RE: The effects of local progesterone on stilbestrol-associated vaginal adenosis.
- Herbst AL, Poskanzer DC, Robboy SJ, Friedlander L, Scully RE: Prenatal exposure to stilbestrol. A prospective comparison of exposed female offspring with unexposed control.
- Stafl A, Mattingly RF, Foley DV, Fetherston WC: Clinical diagnosis of vaginal adenosis.
- Sherman AL, Goldrath M, Berlin A, et al: Cervical-vaginal adenosis after
- Gall, Kirman B, Stern J: Hormonal pregnancy tests and congenital malformation.
- Levy EP, Cohen A, Fraser FC: Hormone treatment during pregnancy and congenital heart defects.
- Nora JJ, Nora AH: Birth defects and oral contraceptives.
- Janerich DT, Piper JM, Glebatis DM: Oral contraceptives and congenital limb-reduction defects.
- Boston Collaborative Drug Surveillance Program: Surgically confirmed gall bladder disease, venous thromboembolism, and breast tumors in relation to post-menopausal estrogen therapy.
- Hoover R, Gray LA, Cole P, MacMahon B: Menopausal estrogens and breast cancer.
- Boston Collaborative Drug Surveillance Program: Oral contraceptives and venous thromboembolic disease, surgically confirmed gall bladder disease, and breast tumors.
- Daniel DG, Campbell H, Turnbull AC: Puerperal thromboembolism and suppression of lactation.
- The Veterans Administration Cooperative Urological Research Group: Carcinoma of the prostate: Treatment comparisons.
- Bailar JC: Thromboembolism and estrogen therapy.
- Blackard CE, Doe RP, Mellinger GT, Byar DP: Incidence of cardiovascular disease and death in patients receiving diethylstilbestrol for carcinoma of the prostate.
- Royal College of General Practitioners: Oral contraception and thromboembolic disease.
- Inman WHW, Vessey MP: Investigation of deaths from pulmonary, coronary, and cerebral thrombosis and embolism in women of childbearing age.
- Vessey MP, Doll R: Investigation of relation between use of oral contraceptives and thromboembolic disease. A further report.
- Sartwell PE, Masi AT, Arthes FG, et al: Thromboembolism and oral contraceptives: An epidemiologic case-control study.
- Collaborative Group for the Study of Stroke in Young Women: Oral contraception and increased risk of cerebral ischemia or thrombosis.
- Collaborative Group for the Study of Stroke in Young Women: Oral contraceptives and stroke in young women: Associated risk factors.
- Mann JI, Inman WHW: Oral contraceptives and death from myocardial infarction.
- Mann JI, Vessey MP, Thorogood M, Doll R: Myocardial infarction in young women with special reference to oral contraceptive practice.
- Inman WHW, Vessey MP, Westerholm B, Engelund A: Thromboembolic disease and the steroidal content of oral contraceptives.
- Stolley PD, Tonascia JA, Tockman MS, et al: Thrombosis with low-estrogen oral contraceptives.
- Vessey MP, Doll R, Fairbairn AS, Glober G: Postoperative thromboembolism and the use of oral contraceptives.
- Greene GR, Sartwell PE: Oral contraceptive use in patients with thromboembolism following surgery, trauma or infection.
- Rosenberg L, Armstrong B, Phil D, Jick H: Myocardial infarction and estrogen therapy in post-menopausal women.
- Coronary Drug Project Research Group: The Coronary Drug Project: Initial findings leading to modifications of its research protocol.
- Baum J, Holtz F, Bookstein JJ, Klein EW: Possible association between benign hepatomas and oral contraceptives.
- Mays ET, Christopherson WM, Mahr MM, Williams HC: Hepatic changes in young women ingesting contraceptive steroids. Hepatic hemorrhage and primary hepatic tumors.
- Edmondson HA, Henderson B, Benton B: Liver-cell adenomas associated with use of oral contraceptives.
- Pfeffer RI, VanDenNoort S: Estrogen use and stroke risk in post-menopausal women.
NDC 0009-0271-01
estradiol cypionate injection, USP

estradiol cypionate
injection, USP