Brand Name
Rapaflo
Generic Name
Silodosin
View Brand Information FDA approval date: March 23, 2009
Classification: alpha-Adrenergic Blocker
Form: Capsule
What is Rapaflo (Silodosin)?
Silodosin capsules, an alpha-1 adrenergic receptor antagonist, is indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia . Silodosin capsule is not indicated for the treatment of hypertension. Silodosin capsule, a selective alpha-1 adrenergic receptor antagonist, is indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia . Silodosin capsule is not indicated for the treatment of hypertension.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
RAPAFLO (silodosin)
1INDICATIONS AND USAGE
RAPAFLO
2DOSAGE FORMS AND STRENGTHS
The 8 mg capsules are white, opaque, hard #1 gelatin capsules imprinted with “WATSON 152” in green on the cap and “8 mg” in green on the body.
The 4 mg capsules are white, opaque, hard #3 gelatin capsules imprinted with “WATSON 151” in gold on the cap and “4 mg” in gold on the body.
3CONTRAINDICATIONS
- Severe renal impairment (CCr < 30 mL/min)
- Severe hepatic impairment (Child-Pugh score
- Concomitant administration with strong Cytochrome P450 3A4 (CYP3A4) inhibitors (e.g., ketoconazole, clarithromycin, itraconazole, ritonavir)
- Patients with a history of hypersensitivity to silodosin or any of the ingredients of RAPAFLO
4OVERDOSAGE
RAPAFLO was evaluated at doses of up to 48 mg/day in healthy male subjects. The dose-limiting adverse event was postural hypotension.
Should overdose of RAPAFLO lead to hypotension, support of the cardiovascular system is of first importance. Restoration of blood pressure and normalization of heart rate may be accomplished by maintaining the patient in the supine position. If this measure is inadequate, administration of intravenous fluid should be considered. If necessary, vasopressors could be used, and renal function should be monitored and supported as needed. Dialysis is unlikely to be of significant benefit since silodosin is highly (97%) protein bound.
5DESCRIPTION
RAPAFLO is the brand name for silodosin, a selective antagonist of alpha-1 adrenoreceptors. The chemical name of silodosin is 1-(3-Hydroxypropyl)-5-[(2
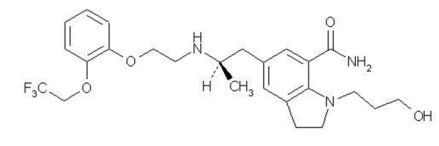
Silodosin is a white to pale yellowish white powder that melts at approximately 105 to 109°C. It is very soluble in acetic acid, freely soluble in alcohol, and very slightly soluble in water.
Each RAPAFLO 8 mg capsule for oral administration contains 8 mg silodosin, and the following inactive ingredients: D-mannitol, magnesium stearate, pregelatinized starch, and sodium lauryl sulfate. The size #1 hard gelatin capsules contain gelatin and titanium dioxide. The capsules are printed with edible ink containing FD&C Blue No. 1 Aluminum Lake and yellow iron oxide.
Each RAPAFLO 4 mg capsule for oral administration contains 4 mg silodosin, and the following inactive ingredients: D-mannitol, magnesium stearate, pregelatinized starch, and sodium lauryl sulfate. The size #3 hard gelatin capsules contain gelatin and titanium dioxide. The capsules are printed with edible ink containing yellow iron oxide.
6HOW SUPPLIED/STORAGE AND HANDLING
White, opaque, hard gelatin 8 mg capsules. Cap is imprinted with “WATSON 152” in green. Body is imprinted with “8 mg” in green. 8 mg capsules are supplied in unit of use HDPE bottles of:
- 30 capsules (
- 90 capsules (
Bottles of 30 and 90 capsules are supplied with child-resistant closures.
White, opaque, hard gelatin 4 mg capsules. Cap is imprinted with “WATSON 151” in gold. Body is imprinted with “4 mg” in gold. 4 mg capsules are supplied in unit of use HDPE bottles of:
- 30 capsules (
Bottles of 30 capsules are supplied with child-resistant closures.
Storage
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F). [See USP controlled room temperature.] Protect from light and moisture.
Keep out of reach of children.
7PATIENT COUNSELING INFORMATION
Advise patients to take RAPAFLO once daily with a meal
Advise patients about the possible occurrence of symptoms related to postural hypotension (such as dizziness), and should be cautioned about driving, operating machinery, or performing hazardous tasks until they know how RAPAFLO will affect them. This is especially important for those with low blood pressure or who are taking antihypertensive medications
Counsel patients on that the most common side effect seen with RAPAFLO is an orgasm with reduced or no semen. This side effect does not pose a safety concern and is reversible with discontinuation of the product
Counsel patients to tell their ophthalmologist about the use of RAPAFLO before cataract surgery or other procedures involving the eyes, even if the patient is no longer taking RAPAFLO
For all medical inquiries contact:
Distributed by:
Under license from:
Product of Japan
© 2020 Allergan. All rights reserved.
For additional information see:
v1.0USPI6142