Generic Name
Mycophenolate Mofetil
Brand Names
CellCept, Myhibbin
FDA approval date: May 03, 1995
Classification: Antimetabolite Immunosuppressant
Form: Injection, Tablet, Powder, Suspension, Capsule
What is CellCept (Mycophenolate Mofetil)?
Mycophenolate Mofetil is indicated for the prophylaxis of organ rejection, in adult and pediatric recipients 3 months of age and older of allogenic kidney [see Clinical Studies (1.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
CellCept (Mycophenolate Mofetil)
WARNING: EMBRYOFETAL TOXICITY, MALIGNANCIES and SERIOUS INFECTIONS
- Use during pregnancy is associated with increased risks of first trimester pregnancy loss and congenital malformations. Avoid if safer treatment options are available. Females of reproductive potential must be counseled regarding pregnancy prevention and planning
- Increased risk of development of lymphoma and other malignancies, particularly of the skin
- Increased susceptibility to bacterial, viral, fungal and protozoal infections, including opportunistic infections and viral reactivation of hepatitis B and C, which may lead to hospitalizations and fatal outcomes
1INDICATIONS AND USAGE
CELLCEPT [mycophenolate mofetil (MMF)] is indicated for the prophylaxis of organ rejection, in adult and pediatric recipients 3 months of age and older of allogeneic kidney
2DOSAGE FORMS AND STRENGTHS
CELLCEPT is available in the following dosage forms and strengths:
3CONTRAINDICATIONS
CELLCEPT is contraindicated in patients with a history of hypersensitivity, including anaphylaxis, to mycophenolate mofetil (MMF), mycophenolic acid (MPA) or any component of the drug product
4ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
- Embryofetal Toxicity
- Lymphomas and Other Malignancies
- Serious Infections
- Blood Dyscrasias: Neutropenia, Pure Red Cell Aplasia
- Gastrointestinal Complications
- Acute Inflammatory Syndrome Associated with Mycophenolate Products
- Hypersensitivity Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
An estimated total of 1557 adult patients received CELLCEPT during pivotal clinical trials in the prevention of acute organ rejection. Of these, 991 were included in the three renal studies, 277 were included in one hepatic study, and 289 were included in one cardiac study. Patients in all study arms also received cyclosporine and corticosteroids.
The data described below primarily derive from five randomized, active-controlled double-blind 12-month trials of CELLCEPT in
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of CELLCEPT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- Embryo-Fetal Toxicity: Congenital malformations and spontaneous abortions, mainly in the first trimester, have been reported following exposure to mycophenolate mofetil (MMF) in combination with other immunosuppressants during pregnancy [see Congenital malformations include:
- Facial malformations: cleft lip, cleft palate, micrognathia, hypertelorism of the orbits
- Abnormalities of the ear and eye: abnormally formed or absent external/middle ear, coloboma, microphthalmos
- Malformations of the fingers: polydactyly, syndactyly, brachydactyly
- Cardiac abnormalities: atrial and ventricular septal defects
- Esophageal malformations: esophageal atresia
- Nervous system malformations: such as spina bifida
- Cardiovascular: Venous thrombosis has been reported in patients treated with CELLCEPT administered intravenously.
- Digestive: Colitis, pancreatitis
- Hematologic and Lymphatic: Bone marrow failure, cases of pure red cell aplasia (PRCA) and hypogammaglobulinemia have been reported in patients treated with CELLCEPT in combination with other immunosuppressive agents [see .
- Immune: Hypersensitivity reactions, including anaphylaxis and angioedema [see , hypogammaglobinemia.
- Infections: Meningitis, infectious endocarditis, tuberculosis, atypical mycobacterial infection, progressive multifocal leukoencephalopathy, BK virus infection, viral reactivation of hepatitis B and hepatitis C, protozoal infections [see .
- Respiratory: Bronchiectasis, interstitial lung disease, fatal pulmonary fibrosis, have been reported rarely and should be considered in the differential diagnosis of pulmonary symptoms ranging from dyspnea to respiratory failure in post-transplant patients receiving CELLCEPT.
- Vascular: Lymphocele
5OVERDOSAGE
Possible signs and symptoms of acute overdose include hematological abnormalities such as leukopenia and neutropenia, and gastrointestinal symptoms such as abdominal pain, diarrhea, nausea, vomiting, and dyspepsia.
The experience with overdose of CELLCEPT in humans is limited. The reported effects associated with overdose fall within the known safety profile of the drug. The highest dose administered to kidney transplant patients in clinical trials has been 4 g/day. In limited experience with heart and liver transplant patients in clinical trials, the highest doses used were 4 g/day or 5 g/day. At doses of 4 g/day or 5 g/day, there appears to be a higher rate, compared to the use of 3 g/day or less, of gastrointestinal intolerance (nausea, vomiting, and/or diarrhea), and occasional hematologic abnormalities, particularly neutropenia
6DESCRIPTION
CELLCEPT (mycophenolate mofetil) is an antimetabolite immunosuppressant. It is the 2-morpholinoethyl ester of mycophenolic acid (MPA), an immunosuppressive agent; inosine monophosphate dehydrogenase (IMPDH) inhibitor.
The chemical name for mycophenolate mofetil (MMF) is 2-morpholinoethyl (E)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate. It has an empirical formula of C
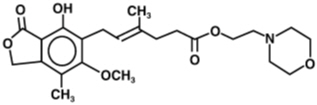
MMF is a white to off-white crystalline powder. It is slightly soluble in water (43 µg/mL at pH 7.4); the solubility increases in acidic medium (4.27 mg/mL at pH 3.6). It is freely soluble in acetone, soluble in methanol, and sparingly soluble in ethanol. The apparent partition coefficient in 1-octanol/water (pH 7.4) buffer solution is 238. The pKa values for MMF are 5.6 for the morpholino group and 8.5 for the phenolic group.
MMF hydrochloride has a solubility of 65.8 mg/mL in 5% Dextrose Injection USP (D5W). The pH of the reconstituted solution is 2.4 to 4.1.
CELLCEPT is available for oral administration as capsules containing 250 mg of MMF, tablets containing 500 mg of MMF, and as a powder for oral suspension which, when reconstituted, contains 200 mg/mL of MMF.
Inactive ingredients in CELLCEPT 250 mg capsules include croscarmellose sodium, magnesium stearate, povidone (K-90) and pregelatinized starch. The capsule shells contain black iron oxide, FD&C blue #2, gelatin, red iron oxide, silicon dioxide, sodium lauryl sulfate, titanium dioxide, and yellow iron oxide.
Inactive ingredients in CELLCEPT 500 mg tablets include croscarmellose sodium, magnesium stearate, microcrystalline cellulose, povidone (K-90), and Opadry
Inactive ingredients in CELLCEPT Oral Suspension include aspartame, citric acid anhydrous, colloidal silicon dioxide, methylparaben, mixed fruit flavor, sodium citrate dihydrate, sorbitol, soybean lecithin, and xanthan gum.
CELLCEPT Intravenous is the hydrochloride salt of MMF. The chemical name for the hydrochloride salt of MMF is 2-morpholinoethyl (E)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate hydrochloride. It has an empirical formula of C
CELLCEPT Intravenous is available as a sterile white to off-white lyophilized powder in single-dose vials containing MMF hydrochloride for administration by intravenous infusion only. Each vial contains 500 mg of mycophenolate mofetil equivalent to 542 mg of mycophenolate mofetil hydrochloride. The inactive ingredients are polysorbate 80, 25 mg, and citric acid, 5 mg. Sodium hydroxide or hydrochloric acid may have been used in the manufacture of CELLCEPT Intravenous to adjust the pH. Reconstitution and dilution with 5% Dextrose Injection USP yields a slightly yellow solution of MMF, 6 mg/mL
7REFERENCES
1. "OSHA Hazardous Drugs." OSHA.
8Instructions for Use CELLCEPT®[SEL-sept] (mycophenolate mofetil) oral suspension
Read this Instructions for Use before you take or give CELLCEPT for the first time and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Important:
- Always use the oral dispenser provided with CELLCEPT Oral Suspension to make sure you measure the right amount of medicine. If your CELLCEPT Oral Suspension does not come with the oral dispenser, contact your pharmacist.
- Call your pharmacist if your oral dispenser is lost or damaged.
- Your pharmacist will write the expiration date on your CELLCEPT Oral Suspension bottle label.
- Ask your doctor or pharmacist if you have any questions or are unsure about how to take or give the right amount of medicine.
- The CELLCEPT Oral Suspension should not be mixed with any type of liquids before taking or giving the dose.
- Do not let the CELLCEPT Oral Suspension come in contact with the skin. If this happens, wash the skin well with soap and water. If the CELLCEPT Oral Suspension gets in the eyes, rinse the eyes with plain water.
- If you spill any CELLCEPT Oral Suspension, wipe it up using paper towels wet with water. Put the child-resistant bottle cap back on the bottle and wipe the outside of the bottle with wet paper towels.
Supplies needed to take or give a dose of CELLCEPT Oral Suspension:
To take or give a dose of CELLCEPT Oral Suspension, you will need the bottle of medicine and the oral dispenser provided with the medicine (
Taking or giving a dose of CELLCEPT Oral Suspension:
How should I store CELLCEPT Oral Suspension?
- Store the CELLCEPT Oral Suspension at room temperature between 59°F to 86°F (15°C to 30°C), for up to 60 days. You can also store the CELLCEPT Oral Suspension in the refrigerator between 36°F to 46°F (2°C to 8°C). )
- Do not freeze.
Keep CELLCEPT Oral Suspension and all medicines out of the reach of children.
Distributed by:
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: August 2022
9PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Carton
NDC 0004-0260-43
CellCept
500 mg
Each tablet contains
Rx only
Attention Pharmacist: Dispense the
500 tablets
Genentech
10235320

10PRINCIPAL DISPLAY PANEL - 250 mg Capsule Bottle Carton
NDC 0004-0259-43
CellCept
250 mg
Each capsule contains
Rx only
Attention Pharmacist: Dispense the
500 capsules
Genentech
10235316

11PRINCIPAL DISPLAY PANEL - 500 mg Vial Carton
NDC 0004-0298-09
CellCept
500 mg
FOR INTRAVENOUS INFUSION ONLY.
Each single-dose vial contains the equivalent of 500 mg mycophenolate mofetil (equivalent to 542 mg of
Attention Pharmacist: Dispense the accompanying Medication Guide to each patient. For additional
Rx only
Genentech
10215811

12PRINCIPAL DISPLAY PANEL - 200 mg/mL Bottle Carton
NDC 0004-0261-29
CellCept
(mycophenolate
mofetil for oral
suspension)
(mycophenolate
mofetil for oral
suspension)
200 mg/mL
Each mL contains 200 mg mycophenolate
Attention Pharmacist: Dispense the accompanying
Rx only
Genentech
10225425
