Brand Name
Radicava
Generic Name
Edaravone
View Brand Information FDA approval date: May 05, 2017
Form: Injection, Kit, Solution
What is Radicava (Edaravone)?
Edaravone injection is indicated for the treatment of amyotrophic lateral sclerosis . Edaravone injection is indicated for the treatment of amyotrophic lateral sclerosis .
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
RADICAVA (EDARAVONE)
1INDICATIONS AND USAGE
RADICAVA and RADICAVA ORS are indicated for the treatment of amyotrophic lateral sclerosis (ALS).
2DOSAGE FORMS AND STRENGTHS
RADICAVA is supplied for intravenous infusion in a single-dose polypropylene bag containing 30 mg of edaravone in 100 mL of clear, colorless aqueous solution.
RADICAVA ORS is supplied as an oral suspension in a multi-dose amber glass bottle 105 mg/5 mL of white to off-white color.
3CONTRAINDICATIONS
RADICAVA and RADICAVA ORS are contraindicated in patients with a history of hypersensitivity to edaravone or any of the inactive ingredients in this product. Hypersensitivity reactions and anaphylactic reactions have occurred
4ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions
- Sulfite Allergic Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In randomized, placebo-controlled trials, 184 patients with ALS were administered RADICAVA 60 mg in treatment cycles for 6 months. The population consisted of Japanese patients who had a median age of 60 years (range 29-75) and were 59% male. Most (93%) of these patients were living independently at the time of screening.
Most Common Adverse Reactions Observed During Clinical Studies
Table 2 lists the adverse reactions that occurred in ≥ 2% of patients in the RADICAVA-treated group and that occurred at least 2% more frequently than in the placebo-treated group in randomized placebo-controlled ALS trials. The most common adverse reactions that occurred in ≥10% of RADICAVA-treated patients were contusion, gait disturbance, and headache.
Table 2: Adverse Reactions from Pooled Placebo-Controlled Trials
- a Pooled placebo-controlled studies include two additional studies with 231 additional patients, all using the same treatment regimen [see
Additional Adverse Reactions with RADICAVA ORS
In an open-label study in patients with ALS (n=185) treated with RADICAVA ORS for 6 months, fatigue was observed in 7.6% of patients.
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of RADICAVA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and subcutaneous tissue disorders: Hypersensitivity reactions and anaphylaxis [see
5DESCRIPTION
The active ingredient in RADICAVA and RADICAVA ORS is edaravone, which is a member of the substituted 2-pyrazolin-5-one class. The chemical name of edaravone is [3-methyl-1-phenyl-2-pyrazolin-5-one]. The molecular formula is C
The chemical structure is:

Edaravone is a white crystalline powder with a melting point of 129.7°C. It is freely soluble in acetic acid, methanol, or ethanol and slightly soluble in water or diethyl ether.
RADICAVA injection is a clear, colorless liquid provided as a sterile solution.
RADICAVA injection is supplied for intravenous infusion in a polypropylene bag containing 30 mg edaravone in 100 mL isotonic, sterile, aqueous solution, which is further overwrapped with polyvinyl alcohol (PVA) secondary packaging. The overwrapped package also contains an oxygen absorber and oxygen indicator to minimize oxidation. Each bag contains the following inactive ingredients: L-cysteine hydrochloride hydrate (10 mg), sodium bisulfite (20 mg). Sodium chloride is added for isotonicity and phosphoric acid and sodium hydroxide are added to adjust to pH 4.
RADICAVA ORS (edaravone) oral suspension is a white to off-white color, opaque, homogenous suspension containing 105 mg of edaravone per 5 mL of suspension.
RADICAVA ORS contains the following inactive ingredients: L-cysteine hydrochloride hydrate, polyvinyl alcohol, simethicone emulsion, sodium bisulfite, sorbitol, and xanthan gum. Phosphoric acid and sodium hydroxide are added to adjust to pH 4.
6CLINICAL STUDIES
The efficacy of RADICAVA ORS is based on a bioavailability study comparing RADICAVA and RADICAVA ORS
The efficacy of RADICAVA for the treatment of ALS was established in a 6-month, randomized, placebo- controlled, double-blind study conducted in Japanese patients with ALS who were living independently and met the following criteria at screening:
- Functionality retained most activities of daily living (defined as scores of 2 points or better on each individual item of the ALS Functional Rating Scale – Revised [ALSFRS-R; described below])
- Normal respiratory function (defined as percent-predicted forced vital capacity values of [%FVC] ≥80%)
- Definite or Probable ALS based on El Escorial revised criteria
- Disease duration of 2 years or less
The study enrolled 69 patients in the RADICAVA arm and 68 in the placebo arm. Baseline characteristics were similar between these groups, with over 90% of patients in each group being treated with riluzole.
RADICAVA was administered as an intravenous infusion of 60 mg given over a 60-minute period according to the following schedule:
- An initial treatment cycle with daily dosing for 14 days, followed by a 14-day drug-free period (Cycle 1)
- Subsequent treatment cycles with daily dosing for 10 days out of 14-day periods, followed by 14-day drug-free periods (Cycles 2-6).
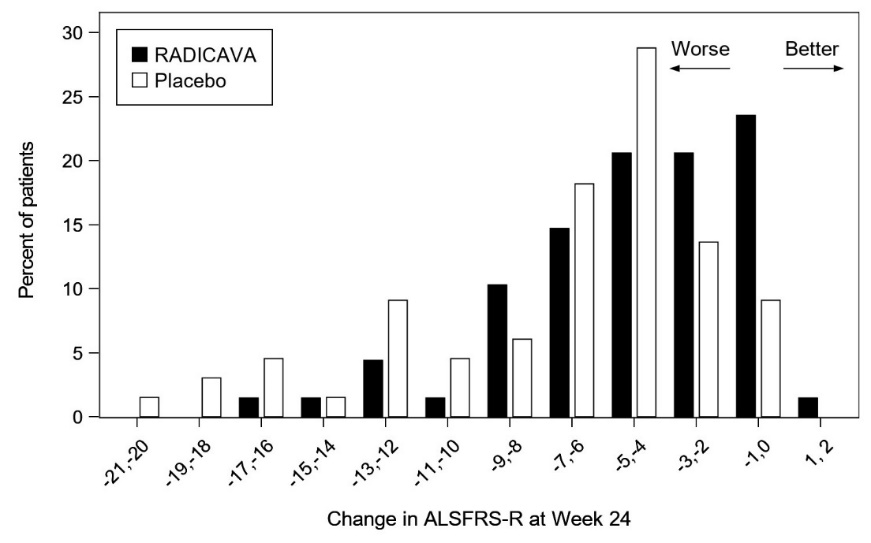
The primary efficacy endpoint was a comparison of the change between treatment arms in the ALSFRS-R total scores from baseline to Week 24. The ALSFRS-R scale consists of 12 questions that evaluate the fine motor, gross motor, bulbar, and respiratory function of patients with ALS (speech, salivation, swallowing, handwriting, cutting food, dressing/hygiene, turning in bed, walking, climbing stairs, dyspnea, orthopnea, and respiratory insufficiency). Each item is scored from 0-4, with higher scores representing greater functional ability. The decline in ALSFRS-R scores from baseline was significantly less in the RADICAVA-treated patients as compared to placebo (see Table 3). The distribution of change in ALSFRS-R scores from baseline to Week 24 by percent of patients is shown in Figure 1.
Table 3: Analysis of Change from Baseline to Week 24 in ALSFRS-R Scores
Figure 1 Distribution of Change from Baseline to Week 24 in ALSFRS-R Scores
7PATIENT COUNSELING INFORMATION
Advise the patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Hypersensitivity Reactions
Advise patients to seek immediate medical care if they experience signs or symptoms of a hypersensitivity reaction
Sulfite Allergic Reactions
Advise patients about potential for sulfite sensitivity. Inform patients that RADICAVA and RADICAVA ORS contain sodium bisulfite, which may cause allergic type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes, and to seek immediate medical care if they experience these signs or symptoms
Pregnancy and Breastfeeding
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during RADICAVA or RADICAVA ORS therapy
Advise patients to notify their healthcare provider if they intend to breastfeed or are breastfeeding an infant
RADICAVA ORS Administration
Advise patients to take RADICAVA ORS in the morning on an empty stomach. Instruct patients to fast 8 hours before each dose if they consume a high-fat meal, 4 hours before each dose if they consume a low-fat meal, or 2 hours before each dose if they consume a caloric supplement
Advise caregivers, if administering RADICAVA ORS via feeding tube, to use a catheter-tip syringe and flush the feeding tube with approximately 1 ounce (30 mL) of water before and after use
Marketed and distributed by:
Mitsubishi Tanabe Pharma America, Inc., a US subsidiary of Mitsubishi Tanabe Pharma Corporation
Jersey City, NJ 07310
RADICAVA and RADICAVA ORS are registered trademark of Mitsubishi Tanabe Pharma Corporation
© 2022 Mitsubishi Tanabe Pharma Corporation
- 22022-2 Iss. 11/2022
7.1Information for Patients
- This Patient Information has been approved by the U.S. Food and Drug Administration 22022-1 Issued: 05/2022
7.2Instructions for Use
INSTRUCTIONS FOR USE
RADICAVA (ra di ká vah) ORS
(edaravone)
oral suspension
- Read this “Instructions for Use” before you take RADICAVA ORS for the first time and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
- Important information about measuring RADICAVA ORS: Always measure your prescribed dose of RADICAVA ORS using the oral syringe provided. Ask your healthcare provider or pharmacist who provided the medicine any questions you have about how to measure your prescribed dose. If you miss a dose, do not take 2 doses of RADICAVA ORS the next day. Do not take a dose of RADICAVA ORS on days 15 through 28.
How to prepare RADICAVA ORS: Keep this Instructions for Use handy when preparing the treatment. - If your healthcare provider prescribes a Starter Kit, you will receive 2 bottles of RADICAVA ORS. Each bottle will contain 35 mL of RADICAVA ORS for a total of 70 mL to be used for your first treatment cycle of 14 days.
- If you were not prescribed the Starter Kit, for each treatment cycle you will receive 1 bottle of RADICAVA ORS that contains a total of 50 mL of RADICAVA ORS. After the first treatment cycle, RADICAVA ORS is to be used for 10 days out of 14 day periods.
- Only use the bottle adapter and the 2 reusable 5 mL oral syringes provided with the bottle.
How to store RADICAVA ORS:
- Store RADICAVA ORS upright at room temperature between 68°F to 77°F (20°C to 25°C). Protect from light.
- Opening the bottle:
- When you open the bottle of RADICAVA ORS for the first use, write the date you open the bottle on the bottle label.
- After opening the bottle of RADICAVA ORS, use within 15 days.
- After a bottle of RADICAVA ORS has been opened and used, a white crust may form on the neck or on the side of the bottle. This is due to normal use and RADICAVA ORS can continue to be uses as prescribed.
- Keep bottle tightly closed between each use.
- Throw away (discard) any RADICAVA ORS that is not used within 15 days after opening the bottle or within 30 days from the date of shipment shown on the carton pharmacy label, whichever happens first. Ask your pharmacist how to properly throw away (discard) RADICAVA ORS you are no longer able to use.
- Keep RADICAVA ORS and all medicines out of the reach of children.
Each RADICAVA ORS carton contains:
- 1 RADICAVA ORS bottle
- 1 bottle adapter
- 2 (5 mL) reusable oral syringes
Use a new 5 mL oral syringe and bottle adapter when using a new bottle of RADICAVA ORS (see Figure A)
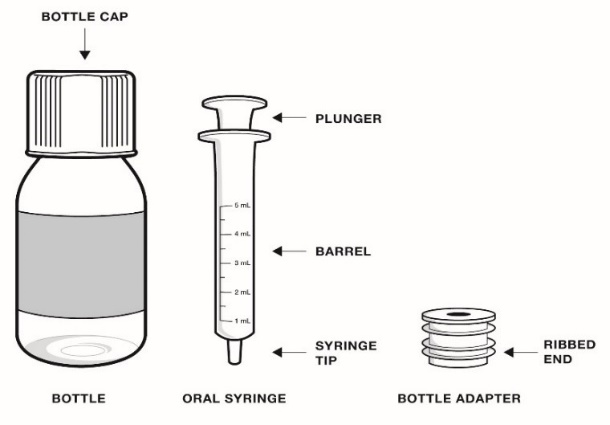
Figure A
Important information:
- Keep these instructions for future use.
- Do not share RADICAVA ORS with anyone else.
- Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
- People who have problems using their hands may need assistance to draw up and give the correct dose of RADICAVA ORS.
How to take RADICAVA ORS:
Take RADICAVA ORS as prescribed by your healthcare provider.
- Dosing Information:
- RADICAVA ORS has 2 different dosing schedules:
- For the first treatment cycle, you will take RADICAVA ORS every day for 14 days, followed by 14 days without the medicine.
- For the cycles after the first treatment cycle, you will take RADICAVA ORS daily for 10 out of 14 days, followed by 14 days without the medicine.
- How RADICAVA ORS will be provided:
- If your healthcare provider prescribes the Starter Kit, you will receive 2 bottles of RADICAVA ORS. Each bottle contains 35 mL of RADICAVA ORS for a total of 70 mL to deliver 14 doses.
- If you were not prescribed the starter kit, for each treatment cycle, you will receive 1 bottle of RADICAVA ORS that contains a total of 50 mL of RADICAVA ORS to deliver 10 doses.
- Fasting Information:
- Do not eat or drink anything 8 hours before each dose of RADICAVA ORS if you eat a high-fat meal.
- Do not eat or drink anything 4 hours before each dose of RADICAVA ORS if you eat a low-fat meal.
- Do not eat or drink anything 2 hours before each dose of RADICAVA ORS if you take a calorie supplement.
- You should wait at least 1 hour after taking your medicine before eating or drinking anything except water.
Step 1: Before each use of RADICAVA ORS: Before opening the bottle, turn it upside down (invert) and shake vigorously up and down for at least 30 seconds (see Figure B). Look at the liquid medicine to make sure it is mixed well. If you see any small pieces at the bottom of the bottle, invert the bottle and shake it up and down for another 30 seconds or until you can no longer see the pieces at the bottom of the bottle (see Figure B). If the liquid medicine all looks the same, you can proceed to Step 2.
Shake up and down for at least 30 seconds
Figure B
Step 2: Open the bottle by firmly pressing down on the bottle cap and turning it counterclockwise (to the left). Place the open bottle upright on a flat surface. Do not throw away the bottle cap; you will need to replace it after taking each dose.
First time use of a bottle only. This must only be done 1 time for each bottle. Insert the ribbed end of the bottle adapter into the bottle by firmly pressing it in as far as it will go (see Figure C). Do not remove the bottle adapter.
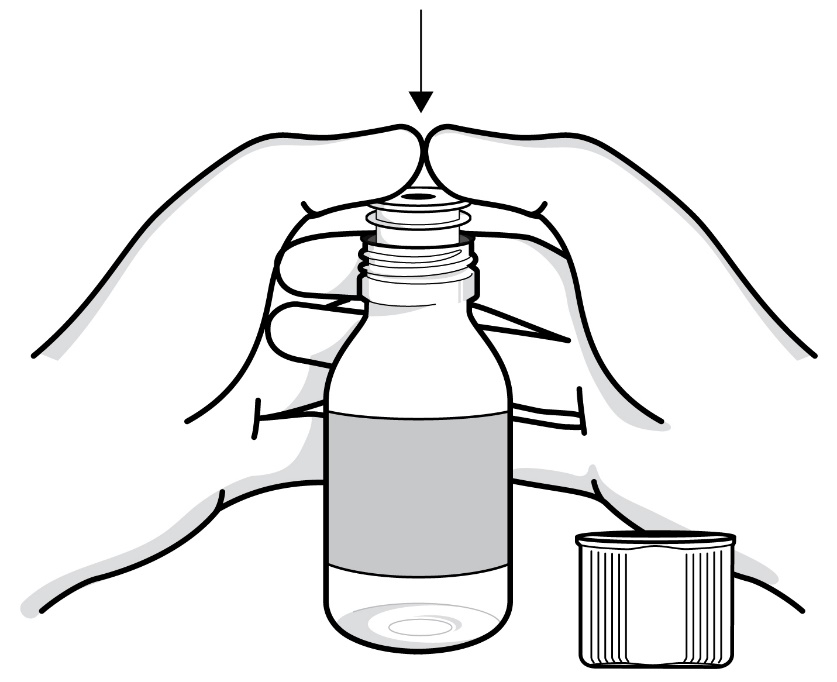
Figure C
Step 3: Remove the oral syringe from plastic wrap and make sure the plunger is inserted all the way into the barrel. Push the oral syringe plunger toward its tip to remove excess air (see Figure D). Insert the oral syringe into the opening of the bottle adapter until it is firmly in place (see Figure E). Turn the bottle upside down and slowly pull the plunger to remove a small amount of liquid (see Figure F).

- Figure D

Figure E

Figure F
Step 4: Keep the bottle upside down and pull the plunger until it goes up to the last line (5 mL) (see Figure G). While keeping the plunger in the same position, turn the bottle upright, and place it carefully on a flat surface. Remove the oral syringe by gently twisting or pulling it out from the bottle (see Figure H). Check the amount of medicine again before moving on to the next step (see Figure I).
Note:
Note: If the dose is not correct, insert the oral syringe tip firmly into the bottle adapter. Push the plunger all the way in so that the medicine flows back into the bottle. Turn the bottle upside down. Repeat Step 4.
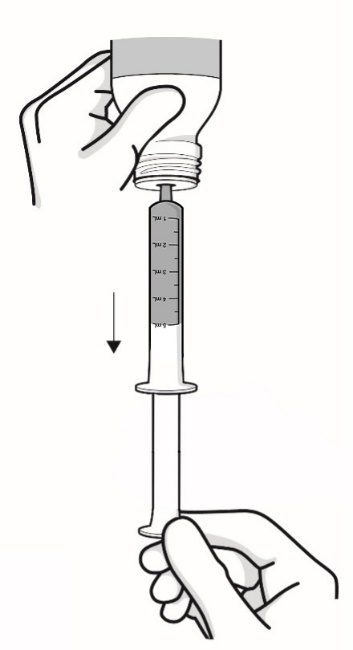
Figure G

Figure H

Figure I
Step 5: Place the tip of the oral syringe in the mouth and aim towards the inside of the cheek. Slowly press down on the plunger until the oral syringe is empty. Swallow all of the medicine (see Figure J). If needed, you can use up to 8 ounces or 1 cup of water to help swallow the medicine.
Note: It is normal for a small amount of medicine to remain in the tip of the syringe after taking.
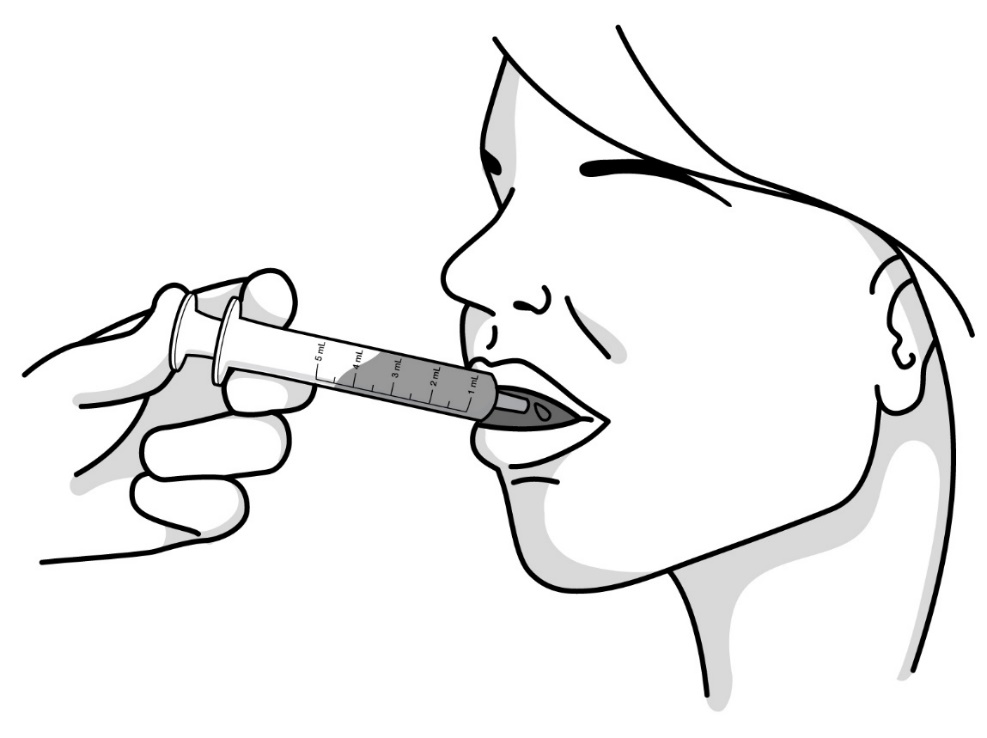
Figure J
Step 6: Leave the bottle adapter inside the bottle. Place the bottle cap on the bottle and turn the cap clockwise (to the right). Keep bottle tightly closed between each use (see Figure K).
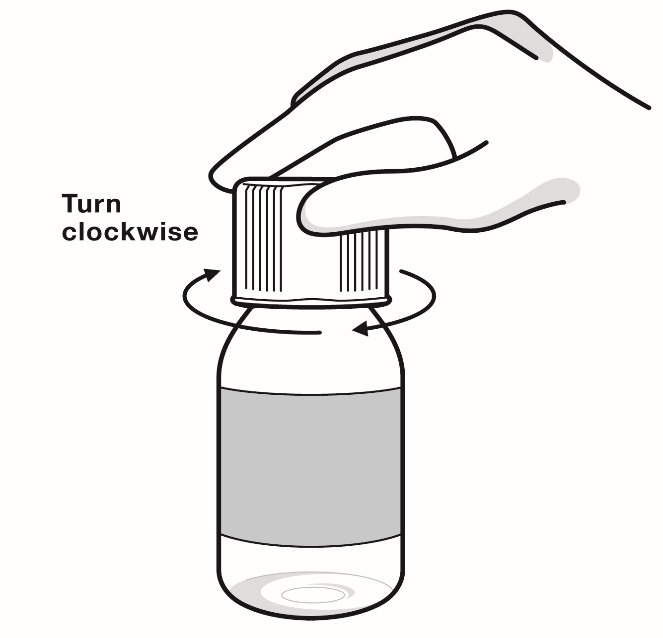
Figure K
Step 7: Remove the plunger from the oral syringe barrel by pulling the plunger and the barrel away from each other. Rinse the oral syringe (plunger and barrel) with water only. Allow it to air dry.
Figure L
Step 8: When the oral syringe (plunger and barrel) is dry, put the plunger back into the oral syringe barrel. Do not throw away the oral syringe. Store the syringe in a clean, dry place.
You must complete Steps 1 through 4 under "How to take RADICAVA ORS" before starting Step 9 under "How to take a dose of RADICAVA ORS oral suspension through a feeding tube."
How to take a dose of RADICAVA ORS oral suspension through a feeding tube:
Step 9: Using a catheter-tip syringe, flush the feeding tube with 1 ounce (30 mL) of water (see Figure M).
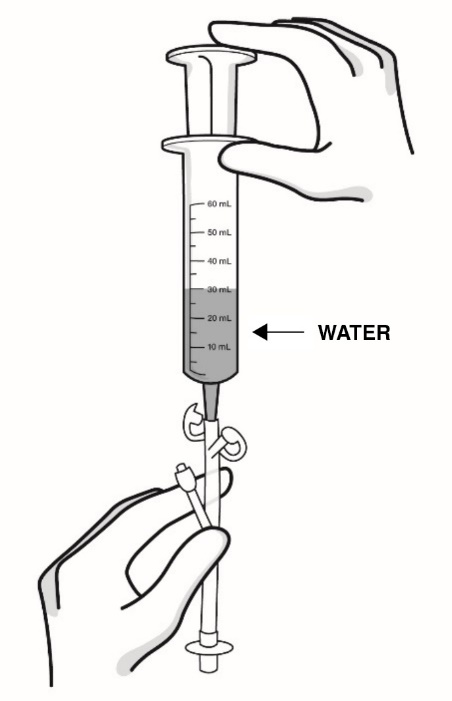
Figure M
Step 10: Place the oral syringe provided (containing the 5 mL of RADICAVA ORS) into the feeding tube. Slowly push down the plunger until the oral syringe is empty (see Figure N).
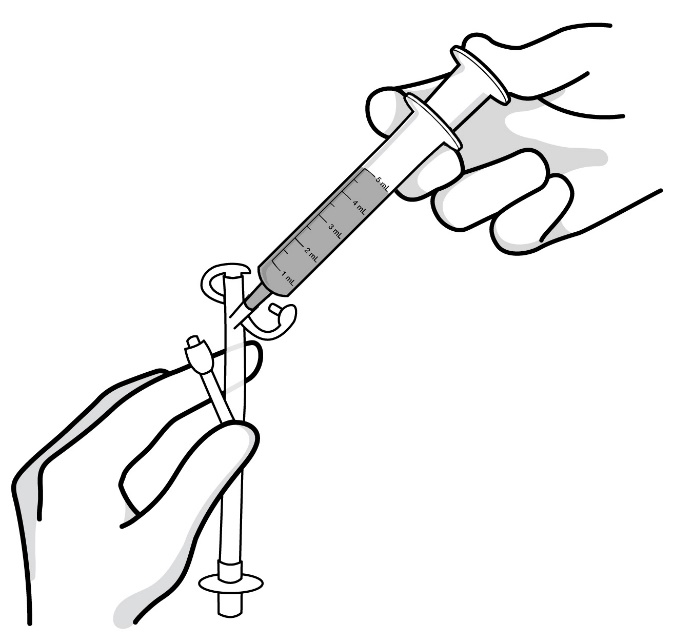
Figure N
Step 11: Using a catheter-tip syringe, flush the feeding tube with 1 ounce (30 mL) of water after taking dose of RADICAVA ORS (see Figure O).
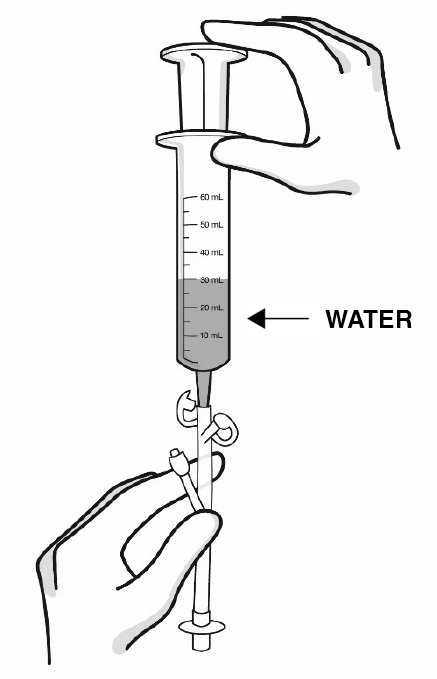
Figure O
Step 12: Leave the adapter in the bottle. Place the bottle cap on the bottle and turn it clockwise (to the right) to close the bottle. Keep the bottle tightly closed between each use (see Figure P).
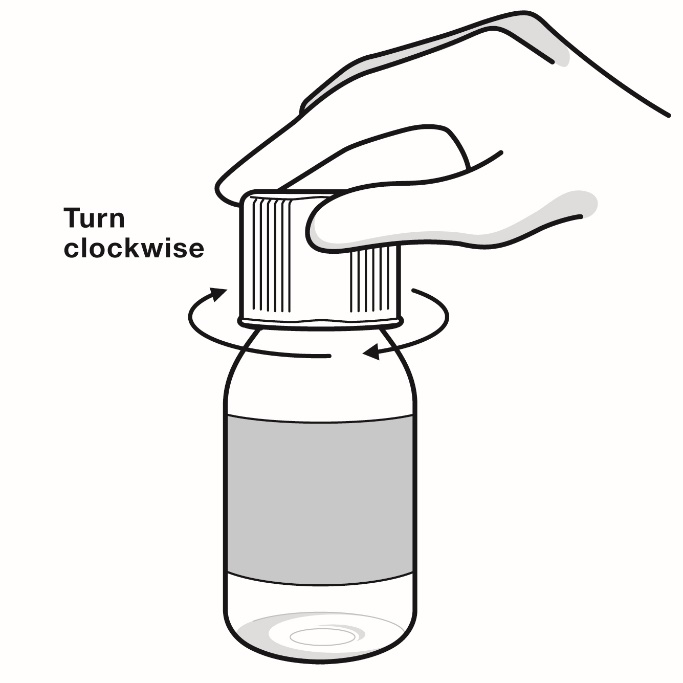
Figure P
Step 13: Remove the plunger from the oral syringe barrel by pulling the plunger and barrel away from each other. Rinse the oral syringe (plunger and barrel) with water only (see Figure Q). Allow it to air dry.
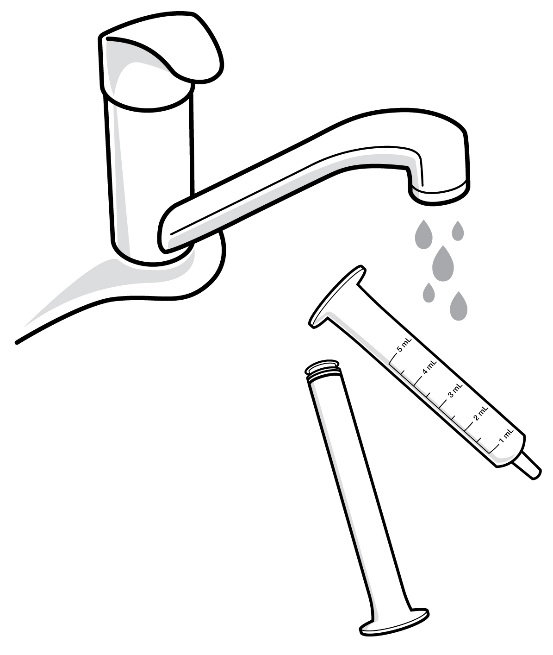
Figure Q
Step 14: When the oral syringe (plunger and barrel) are dry, put the plunger back into the oral syringe barrel. Do not throw away the oral syringe. Store the oral syringe in a clean, dry place.
Marketed and distributed by:
Mitsubishi Tanabe Pharma America, Inc., a US subsidiary of Mitsubishi Tanabe Pharma Corporation
Jersey City, NJ 07310
For more information, go to www.Radicava.com or call 1- 888-292-0058.
RADICAVA ORS®is a registered trademark of Mitsubishi Tanabe Pharma Corporation
© 2021 Mitsubishi Tanabe Pharma Corporation. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. 22022-1
Issued: 05/2022
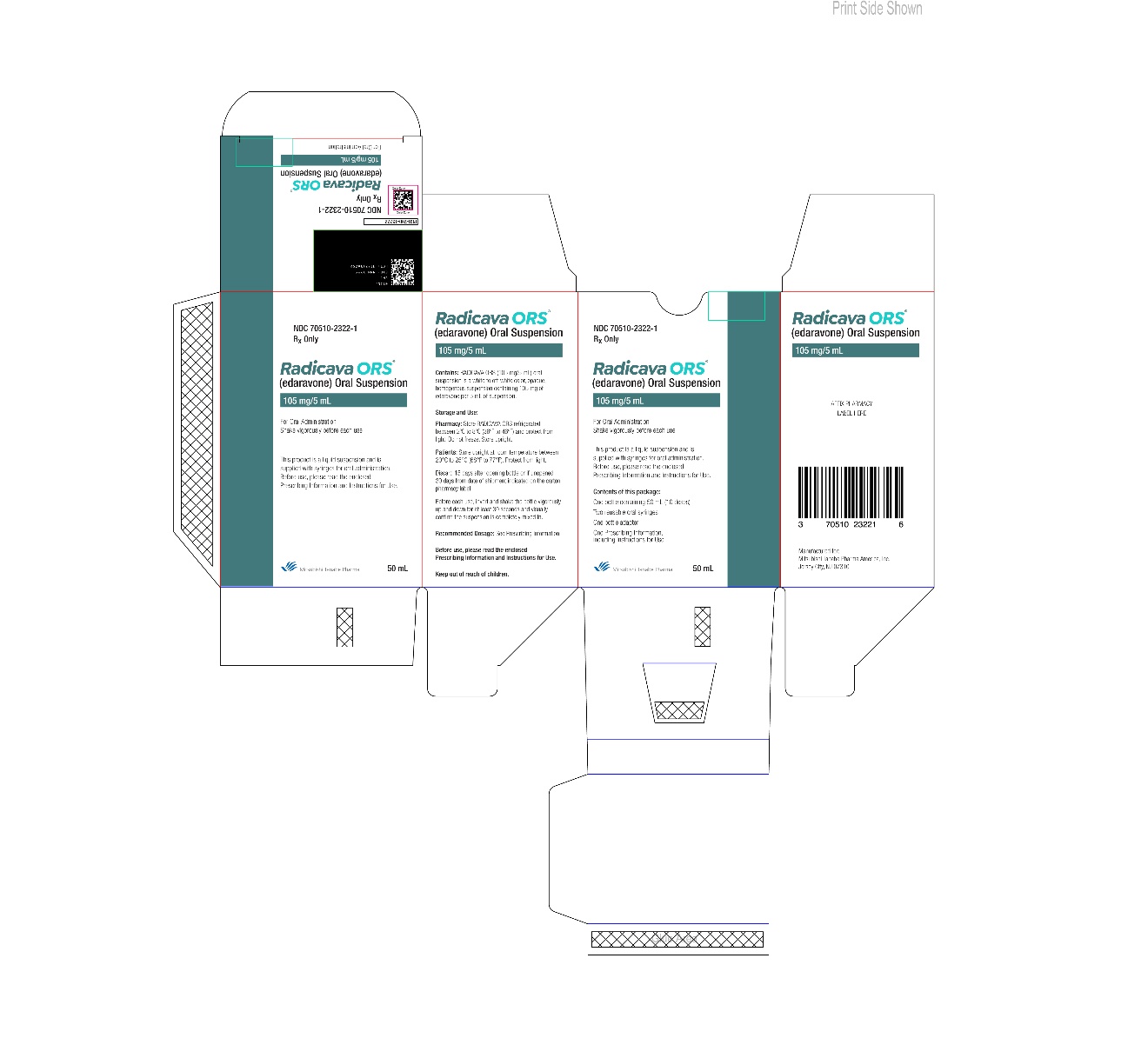
8PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

9PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
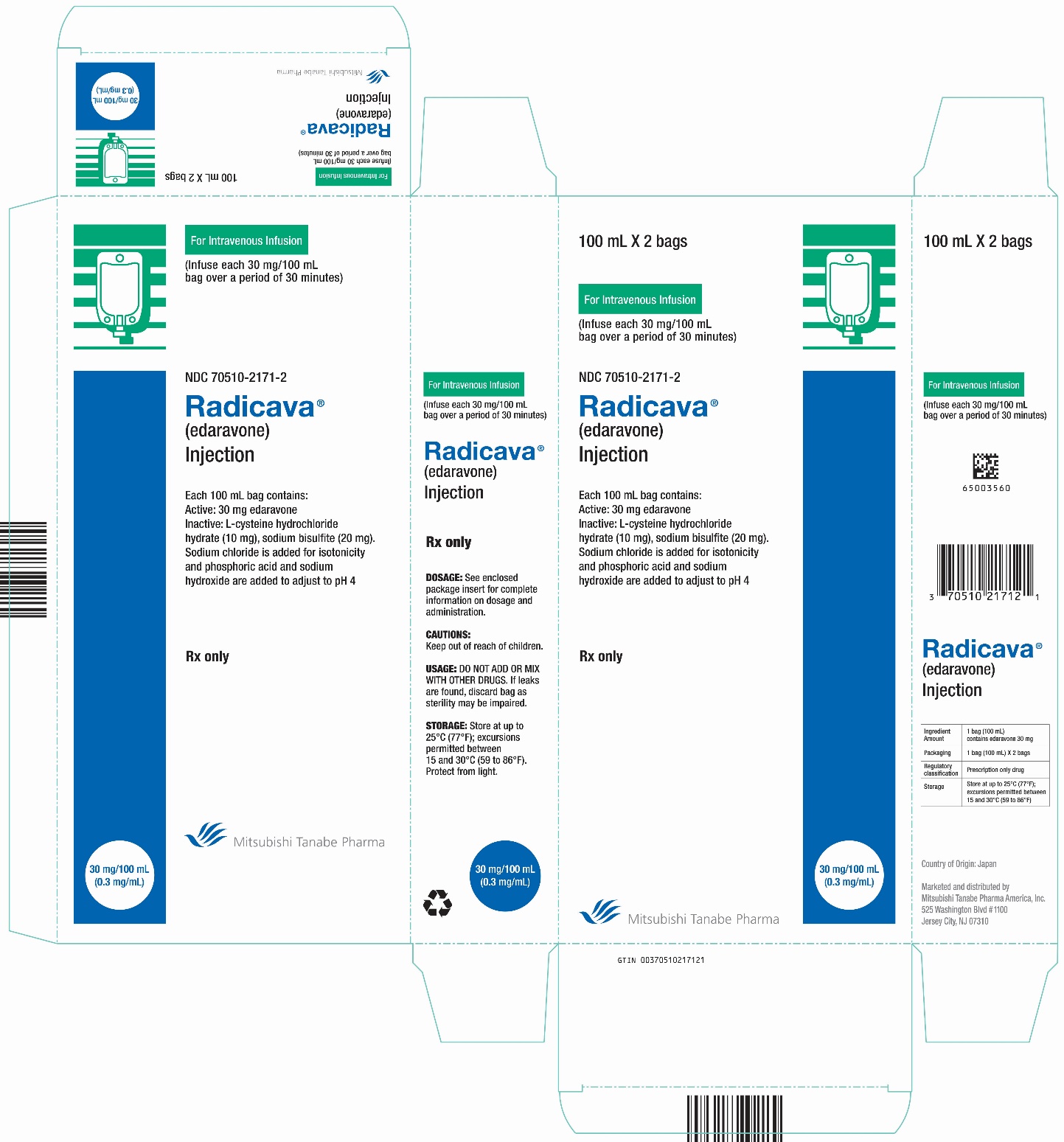
10PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

11PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

12PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

13PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
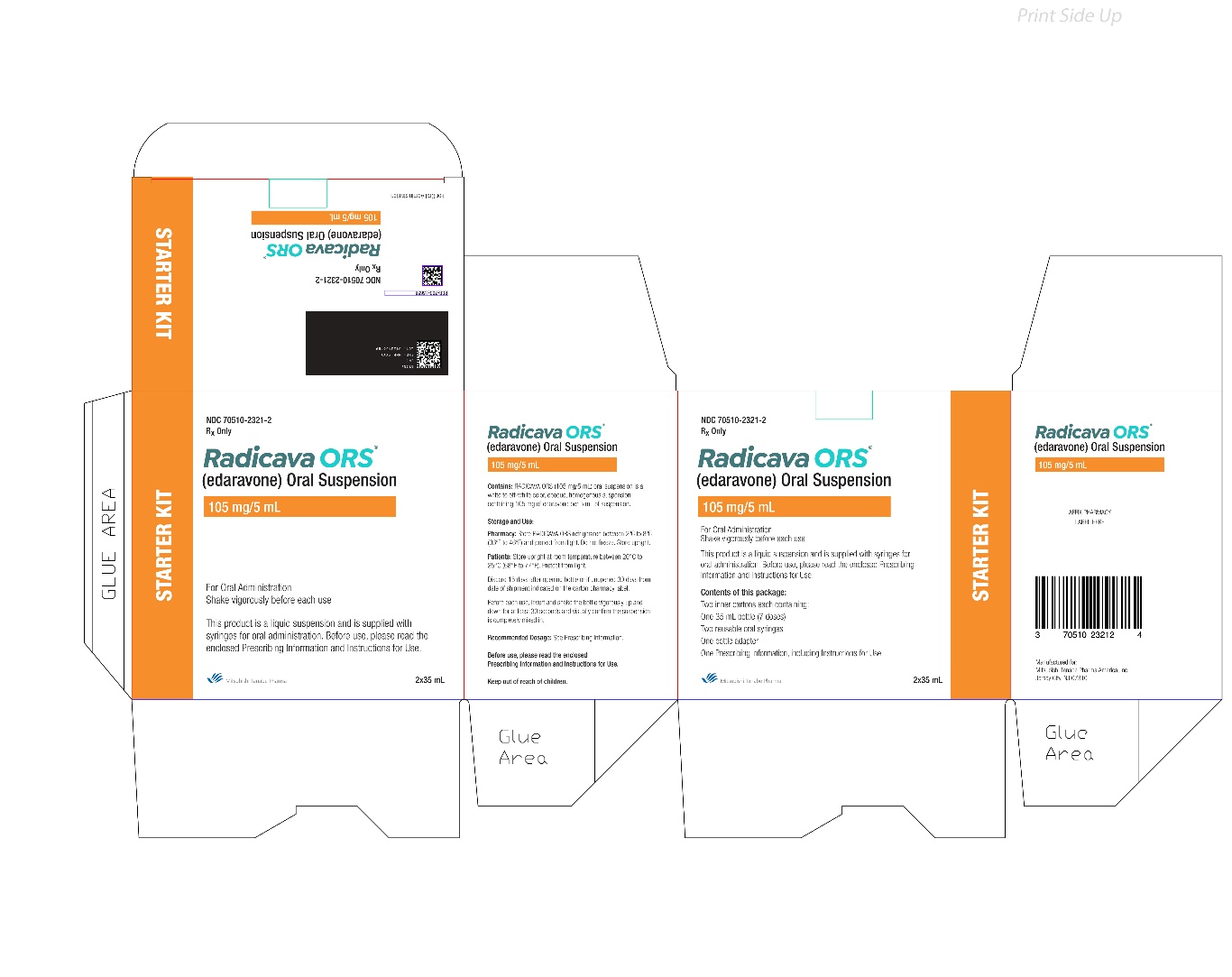
14PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

15PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

16PACKAGE/LABEL PRINCIPAL DISPLAY PANEL