Brand Name
Hympavzi
Generic Name
Marstacimab-Hncq
View Brand Information FDA approval date: November 05, 2024
Form: Injection
What is Hympavzi (Marstacimab-Hncq)?
HYMPAVZI is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older with: hemophilia A without factor VIII inhibitors, or, hemophilia B without factor IX inhibitors. HYMPAVZI is a tissue factor pathway inhibitor antagonist indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older with:, hemophilia A without factor VIII inhibitors, or, hemophilia B without factor IX inhibitors.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
HYMPAVZI (marstacimab-hncq)
1INDICATIONS AND USAGE
HYMPAVZI is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients 12 years of age and older with:
- hemophilia A (congenital factor VIII deficiency) without factor VIII inhibitors, or
- hemophilia B (congenital factor IX deficiency) without factor IX inhibitors.
2DOSAGE FORMS AND STRENGTHS
HYMPAVZI (marstacimab‑hncq) is a clear and colorless to light yellow solution available as:
Prefilled Syringe
- Injection: 150 mg/mL in a single-dose prefilled syringe
Prefilled Pen
- Injection: 150 mg/mL in a single-dose prefilled pen
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Thromboembolic Events
- Hypersensitivity
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of HYMPAVZI was evaluated in adolescent and adult patients with severe hemophilia A or B without inhibitors (coagulation factor activity <1%) enrolled in the BASIS study
A serious adverse reaction of peripheral swelling occurred in one patient.
Table 1 summarizes the adverse reactions reported in ≥3% of patients who received HYMPAVZI prophylaxis.
A serious adverse reaction of venous thrombosis occurred in 0.9% of patients (1/116) treated with HYMPAVZI in the open-label extension study
5DRUG INTERACTIONS
Partial Thromboplastin Time (aPTT) and Prothrombin Time (PT)No clinically significant differences in standard measures of coagulation including activated partial thromboplastin time (aPTT) and prothrombin time (PT) were observed following marstacimab‑hncq therapy.
6DESCRIPTION
Marstacimab‑hncq is a tissue factor pathway inhibitor (TFPI) antagonist, human monoclonal immunoglobulin G Type 1 (IgG1) antibody. Marstacimab‑hncq is produced by Chinese hamster ovary (CHO) cells by recombinant DNA technology and has a molecular mass of approximately 146 kDa.
HYMPAVZI (marstacimab‑hncq) injection is supplied as a sterile, preservative-free solution for subcutaneous administration. The drug product is supplied as either a single-dose 150 mg/mL prefilled syringe or as a single‑dose 150 mg/mL prefilled pen. The solution of marstacimab‑hncq is clear and colorless to light yellow with a pH of 5.8.
Each 150 mg/mL prefilled syringe or prefilled pen delivers 1 mL of HYMPAVZI. Each 1 mL of HYMPAVZI contains 150 mg of marstacimab‑hncq, and the inactive ingredients edetate disodium (0.05 mg), histidine (1.12 mg), L-histidine monohydrochloride (2.67 mg), polysorbate 80 (0.2 mg), and sucrose (85 mg), in Water for Injection, USP.
7PATIENT COUNSELING INFORMATION
- Advise the patient and/or caregivers to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Ensure that patients and caregivers who will administer HYMPAVZI receive appropriate training and instruction on the proper storage, use and handling of HYMPAVZI from a healthcare professional.
Thromboembolic EventsInform patients and/or caregivers that HYMPAVZI increases coagulation potential. Discuss the appropriate dosing of concomitant agents such as FVIII or FIX with the patient prior to starting on HYMPAVZI prophylaxis [see . Advise the patient to discontinue HYMPAVZI and seek immediate medical attention if any signs or symptoms of thromboembolism occur.
HypersensitivityInform patients and/or caregivers that hypersensitivity reactions such as rash and pruritus are possible. Advise patients to discontinue HYMPAVZI and seek immediate emergency treatment if a severe hypersensitivity reaction occurs [see .
PregnancyAdvise female patients of reproductive potential to use effective contraception during treatment with HYMPAVZI and for 2 months after the last dose. Advise patients to report known pregnancies [see
This product’s labeling may have been updated. For the most recent prescribing information, please visit
For medical information about HYMPAVZI, please visit

US License No. 2001
Distributed by
Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
LAB-1556-3.0
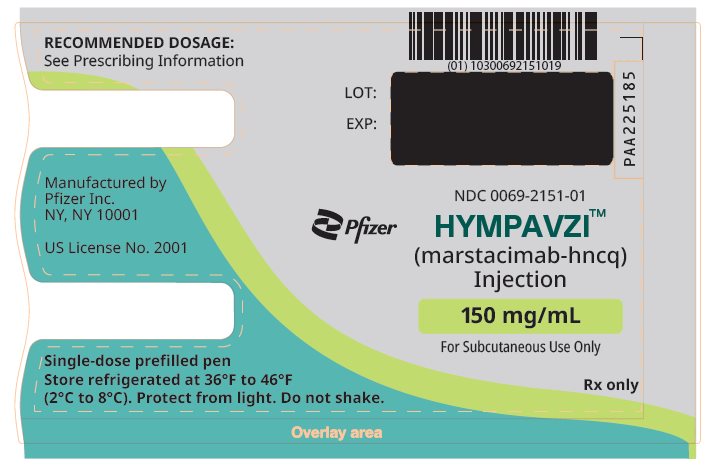
8PRINCIPAL DISPLAY PANEL – 150 mg/mL Prefilled Pen
NDC 0069-2151-01
Pfizer
HYMPAVZI
150 mg/mL
For Subcutaneous Use Only
Rx only
9PRINCIPAL DISPLAY PANEL – 150 mg/mL Prefilled Pen Carton
NDC 0069-2151-01
1 single-dose prefilled pen
Pfizer
HYMPAVZI
150 mg/mL
For Subcutaneous Use Only
This carton contains:
Keep out of reach of children.
Rx only

10PRINCIPAL DISPLAY PANEL - 150 mg/mL Prefilled Syringe
NDC 0069-1510-01
Single-Dose
HYMPAVZI
150 mg/mL
For Subcutaneous Use Only
Rx only
Mfg. by Pfizer Inc.
US Lic. No. 2001

11PRINCIPAL DISPLAY PANEL - 150 mg/mL Prefilled Syringe Carton
NDC 0069-1510-01
1 single-dose prefilled syringe
Pfizer
HYMPAVZI
150 mg/mL
For Subcutaneous Use Only
This carton contains:
Keep out of reach of children.
Rx only
