Generic Name
Cypionate
Brand Names
Depo-Estradiol, Depo-Testosterone, Azmiro
FDA approval date: July 25, 1979
Classification: Androgen
Form: Injection, Kit
What is Depo-Estradiol (Cypionate)?
Testosterone Cypionate Injection is indicated for replacement therapy in the male in conditions associated with symptoms of deficiency or absence of endogenous testosterone. Primary hypogonadism - testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome; or orchidectomy. Hypogonadotropic hypogonadism - gonadotropin or LHRH deficiency, or pituitary-hypothalamic injury from tumors, trauma, or radiation. Safety and efficacy of Testosterone Cypionate Injection in men with “age-related hypogonadism” have not been established.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Depo-Estradiol (estradiol cypionate)
1DESCRIPTION
DEPO-Estradiol Injection contains estradiol cypionate for intramuscular use. Each mL contains:
5 mg/mL—5 mg estradiol cypionate, 5.4 mg chlorobutanol anhydrous (chloral derivative) added as preservative; in 913 mg cottonseed oil.
Warning: Chlorobutanol may be habit forming. The structural formula is represented below:
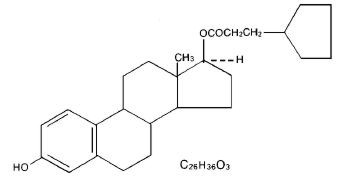
DEPO-Estradiol contains an oil soluble ester of estradiol 17β. The chemical name for estradiol cypionate is estradiol 17-cyclopentanepropionate.
2CLINICAL PHARMACOLOGY
Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than its metabolites, estrone and estriol, at the receptor level.
The primary source of estrogen in normally cycling adult women is the ovarian follicle, which secretes 70 to 500 mcg of estradiol daily, depending on the phase of the menstrual cycle. After menopause, most endogenous estrogen is produced by conversion of androstenedione, secreted by the adrenal cortex, to estrone by peripheral tissues. Thus, estrone and the sulfate conjugated form, estrone sulfate, are the most abundant circulating estrogens in postmenopausal women.
Estrogens act through binding to nuclear receptors in estrogen-responsive tissues. To date, two estrogen receptors have been identified. These vary in proportion from tissue to tissue.
Circulating estrogens modulate the pituitary secretion of the gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH), through a negative feedback mechanism. Estrogens act to reduce the elevated levels of these hormones seen in postmenopausal women.
2.1Absorption
When conjugated with aryl and alkyl groups for parenteral administration, the rate of absorption of oily preparations is slowed with a prolonged duration of action, such that a single intramuscular injection of estradiol valerate or estradiol cypionate is absorbed over several weeks.
2.2Distribution
The distribution of exogenous estrogens is similar to that of endogenous estrogens. Estrogens are widely distributed in the body and are generally found in higher concentrations in the sex hormone target organs. Estrogens circulate in the blood largely bound to sex hormone binding globulin (SHBG) and albumin.
2.3Metabolism
Exogenous estrogens are metabolized in the same manner as endogenous estrogens. Circulating estrogens exist in a dynamic equilibrium of metabolic interconversions. These transformations take place mainly in the liver. Estradiol is converted reversibly to estrone, and both can be converted to estriol, which is the major urinary metabolite. Estrogens also undergo enterohepatic recirculation via sulfate and glucuronide conjugation in the liver, biliary secretion of conjugates into the intestine, and hydrolysis in the gut followed by reabsorption. In postmenopausal women, a significant proportion of the circulating estrogens exist as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogens.
2.4Excretion
Estradiol, estrone, and estriol are excreted in the urine along with glucuronide and sulfate conjugates.
2.5Drug Interactions
In vitro and in vivo studies have shown that estrogens are metabolized partially by cytochrome P450 3A4 (CYP3A4). Therefore, inducers or inhibitors of CYP3A4 may affect estrogen drug metabolism. Inducers of CYP3A4 such as St. John's Wort preparations (Hypericum perforatum), phenobarbital, carbamazepine, and rifampin may reduce plasma concentrations of estrogens, possibly resulting in a decrease in therapeutic effects and/or changes in the uterine bleeding profile. Inhibitors of CYP3A4 such as erythromycin, clarithromycin, ketoconazole, itraconazole, ritonavir and grapefruit juice may increase plasma concentrations of estrogens and may result in side effects.
Estrogen drug products administered by non oral routes are not subject to first-pass metabolism, but also undergo significant hepatic uptake, metabolism, and enterohepatic recycling.
3INDICATIONS AND USAGE
DEPO-Estradiol Injection is indicated in the treatment of:
- Moderate to severe
- Hypoestrogenism due to hypogonadism.
4CONTRAINDICATIONS
Estrogens should not be used in individuals with any of the following conditions:
- Undiagnosed abnormal genital bleeding.
- Known or suspected cancer of the breast.
- Known or suspected estrogen-dependent neoplasia.
- Active deep vein thrombosis, pulmonary embolism or history of these conditions.
- Active or recent (e.g., within the past year) arterial thromboembolic disease (e.g., stroke, myocardial infarction).
- Liver dysfunction or disease.
- DEPO-Estradiol should not be used in patients with known hypersensitivity to its ingredients.
- Known or suspected pregnancy. There is no indication for DEPO-Estradiol in pregnancy.
There appears to be little or no increased risk of birth defects in children born to women who have used estrogens and progestins from oral contraceptives inadvertently during early pregnancy. (See
5WARNINGS
See
5.1Cardiovascular disorders
Estrogen and estrogen/progestin therapy have been associated with an increased risk of cardiovascular events such as myocardial infarction and stroke, as well as venous thrombosis and pulmonary embolism (venous thromboembolism or VTE). Should any of these occur or be suspected, estrogens should be discontinued immediately.
Risk factors for arterial vascular disease (e.g., hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (e.g., personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
5.1.1a. Coronary heart disease and stroke
In the Women's Health Initiative (WHI) study, an increase in the number of myocardial infarctions and strokes has been observed in women receiving CE compared to placebo. These observations are preliminary, and the study is continuing. (See
In the CE/MPA substudy of WHI, an increased risk of coronary heart disease (CHD) events (defined as nonfatal myocardial infarction and CHD death) was observed in women receiving CE/MPA compared to women receiving placebo (37 vs. 30 per 10,000 women-years). The increase in risk was observed in year one and persisted.
In the same substudy of WHI, an increased risk of stroke was observed in women receiving CE/MPA compared to women receiving placebo (29 vs. 21 per 10,000 women-years). The increase in risk was observed after the first year and persisted.
In postmenopausal women with documented heart disease (n = 2,763, average age 66.7 years) a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS) treatment with CE/MPA (0.625 mg/2.5 mg per day) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE/MPA did not reduce the overall rate of CHD events in postmenopausal women with established coronary heart disease. There were more CHD events in the CE/MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred and twenty one women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE/MPA group and the placebo group in HERS, HERS II, and overall.
Large doses of estrogen (5 mg conjugated estrogens per day), comparable to those used to treat cancer of the prostate and breast, have been shown in a large prospective clinical trial in men to increase the risks of nonfatal myocardial infarction, pulmonary embolism, and thrombophlebitis.
5.1.2b. Venous thromboembolism (VTE)
In the Women's Health Initiative (WHI) study, in women receiving CE compared to placebo, the risk of VTE (including both DVT and PE) was increased 33% (28 vs. 21 per 10,000 person-years) although only the increased rate of DVT reached statistical significance (p = 0.03). (See CLINICAL PHARMACOLOGY, Clinical Studies.)
In the CE/MPA treatment substudy of WHI, a 2-fold greater rate of VTE, including deep venous thrombosis and pulmonary embolism, was observed in women receiving treatment with CE/MPA compared to women receiving placebo. The rate of VTE was 34 per 10,000 woman-years in the CE/MPA group compared to 16 per 10,000 woman-years in the placebo group. The increase in VTE risk was observed during the first year and persisted.
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
5.2Dementia
In the Women's Health Initiative Memory Study (WHIMS), 4,532 generally healthy postmenopausal women 65 years of age and older were studied, of whom 35% were 70 to 74 years of age and 18% were 75 or older. After an average follow-up of 4 years, 40 women being treated with CE/MPA (1.8%, n= 2,229) and 21 women in the placebo group (0.9%, n= 2,303) received diagnoses of probable dementia. The relative risk for CE/MPA versus placebo was 2.05 (95% confidence interval 1.21 – 3.48), and was similar for women with and without histories of menopausal hormone use before WHIMS. The absolute risk of probable dementia for CE/MPA versus placebo was 45 versus 22 cases per 10,000 women-years. It is unknown whether these findings apply to younger postmenopausal women. (See
5.3Gallbladder disease
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogen has been reported.
5.4Hypercalcemia
Estrogen administration may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If hypercalcemia occurs, use of the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
5.5Visual abnormalities
Retinal vascular thrombosis has been reported in patients receiving estrogens. Discontinue medication pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, estrogens should be permanently discontinued.
5.6Hereditary angioedema
In women with hereditary angioedema, exogenous estrogens may induce or exacerbate symptoms of angioedema.
6ADVERSE REACTIONS
See
The following additional adverse reactions have been reported with estrogens and/or progestin therapy.
- Genitourinary system
Changes in vaginal bleeding pattern and abnormal withdrawal bleeding or flow; breakthrough bleeding, spotting; dysmenorrhea; increase in size of uterine leiomyomata; vaginitis including vaginal candidiasis; change in amount of cervical secretion; changes in cervical ectropion; ovarian cancer; endometrial hyperplasia; endometrial cancer. - Breasts
Tenderness, enlargement pain, nipple discharge, galactorrhea; fibrocystic breast changes; breast cancer. - Cardiovascular
Deep and superficial venous thrombosis; pulmonary embolism; thrombophlebitis; myocardial infarction; stroke; increase in blood pressure. - Gastrointestinal
Nausea, vomiting; abdominal cramps, bloating; cholestatic jaundice; increased incidence of gallbladder disease; pancreatitis, enlargement of hepatic hemangiomas. - Skin
Chloasma or melasma that may persist when drug is discontinued. Erythema multiforme; erythema nodosum; hemorrhagic eruption; loss of scalp hair; hirsutism; pruritus, rash. - Eyes
Retinal vascular thrombosis; steepening of corneal curvature; intolerance to contact lenses. - Central nervous system
Headache, migraine, dizziness; mental depression; chorea; nervousness; mood disturbances; irritability; exacerbation of epilepsy, dementia. - Miscellaneous
Increase or decrease in weight; reduced carbohydrate tolerance; aggravation of porphyria; edema; changes in libido; arthralgias; leg cramps; anaphylactoid/anaphylactic reactions including urticaria and angioedema; hypocalcemia; exacerbation of asthma; increased triglycerides.
7DRUG ABUSE AND DEPENDENCE
Chlorobutanol anhydrous (chloral derivative) added as a preservative may be habit forming.
8OVERDOSAGE
Serious ill effects have not been reported following acute ingestion of large doses of estrogen-containing oral contraceptives by young children. Overdosage of estrogen may cause nausea and vomiting, and withdrawal bleeding may occur in females.
9DOSAGE AND ADMINISTRATION
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Warming and shaking the vial should redissolve any crystals that may have formed during storage at temperatures lower than recommended.
DEPO-Estradiol INJECTION IS FOR INTRAMUSCULAR USE ONLY.
When estrogen is prescribed for a woman with a uterus, progestin should also be initiated to reduce the risk of endometrial cancer. A woman without a uterus does not need progestin. Use of estrogen, alone or in combination with a progestin, should be with the lowest effective dose and for the shortest duration consistent with treatment goals and risks for the individual woman. Patients should be reevaluated periodically as clinically appropriate (e.g., 3-month to 6-month intervals) to determine if treatment is still necessary. (See
- Short-term cyclic use for treatment of moderate to severe vasomotor symptoms, vulval and vaginal atrophy associated with the menopause, the lowest dose and regimen that will control symptoms should be chosen and medication should be discontinued as promptly as possible.
- For treatment of female hypoestrogenism due to hypogonadism 1.5 to 2 mg injected at monthly intervals.
10HOW SUPPLIED
DEPO-Estradiol Injection is available in the following concentration containing per mL:
5 mg estradiol cypionate; also 5.4 mg chlorobutanol anhydrous (chloral deriv.) added as preservative; in 913 mg cottonseed oil— in 5 mL vials, NDC 0009-0271-01.
WARNING: Chlorobutanol may be habit forming.
11REFERENCES
- Ziel HK, Finkle WD: Increased risk of endometrial carcinoma among users of conjugated estrogens.
- Smith DC, Prentice R, Thompson DJ, et al: Association of exogenous estrogen and endometrial carcinoma.
- Mack TM, Pike MC, Henderson BE, et al: Estrogens and endometrial cancer in a retirement community.
- Weiss NS, Szekely DR, Austin DF: Increasing incidence of endometrial cancer in the United States.
- Herbst AL, Ulfelder H, Poskanzer DC: Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women.
- Greenwald P, Barlow JJ, Nasca PC, Burnett WS: Vaginal cancer after maternal treatment with synthetic estrogens.
- Lanier AP, Noller KL, Decker DG, Elveback LR, Kurland LT: Cancer and stilbestrol. A follow-up of 1,719 persons exposed to estrogens
- Herbst AL, Kurman RJ, Scully RE: Vaginal and cervical abnormalities after exposure to stilbestrol
- Herbst AL, Robboy SJ, Macdonald GJ, Scully RE: The effects of local progesterone on stilbestrol-associated vaginal adenosis.
- Herbst AL, Poskanzer DC, Robboy SJ, Friedlander L, Scully RE: Prenatal exposure to stilbestrol. A prospective comparison of exposed female offspring with unexposed control.
- Stafl A, Mattingly RF, Foley DV, Fetherston WC: Clinical diagnosis of vaginal adenosis.
- Sherman AL, Goldrath M, Berlin A, et al: Cervical-vaginal adenosis after
- Gall, Kirman B, Stern J: Hormonal pregnancy tests and congenital malformation.
- Levy EP, Cohen A, Fraser FC: Hormone treatment during pregnancy and congenital heart defects.
- Nora JJ, Nora AH: Birth defects and oral contraceptives.
- Janerich DT, Piper JM, Glebatis DM: Oral contraceptives and congenital limb-reduction defects.
- Boston Collaborative Drug Surveillance Program: Surgically confirmed gall bladder disease, venous thromboembolism, and breast tumors in relation to post-menopausal estrogen therapy.
- Hoover R, Gray LA, Cole P, MacMahon B: Menopausal estrogens and breast cancer.
- Boston Collaborative Drug Surveillance Program: Oral contraceptives and venous thromboembolic disease, surgically confirmed gall bladder disease, and breast tumors.
- Daniel DG, Campbell H, Turnbull AC: Puerperal thromboembolism and suppression of lactation.
- The Veterans Administration Cooperative Urological Research Group: Carcinoma of the prostate: Treatment comparisons.
- Bailar JC: Thromboembolism and estrogen therapy.
- Blackard CE, Doe RP, Mellinger GT, Byar DP: Incidence of cardiovascular disease and death in patients receiving diethylstilbestrol for carcinoma of the prostate.
- Royal College of General Practitioners: Oral contraception and thromboembolic disease.
- Inman WHW, Vessey MP: Investigation of deaths from pulmonary, coronary, and cerebral thrombosis and embolism in women of childbearing age.
- Vessey MP, Doll R: Investigation of relation between use of oral contraceptives and thromboembolic disease. A further report.
- Sartwell PE, Masi AT, Arthes FG, et al: Thromboembolism and oral contraceptives: An epidemiologic case-control study.
- Collaborative Group for the Study of Stroke in Young Women: Oral contraception and increased risk of cerebral ischemia or thrombosis.
- Collaborative Group for the Study of Stroke in Young Women: Oral contraceptives and stroke in young women: Associated risk factors.
- Mann JI, Inman WHW: Oral contraceptives and death from myocardial infarction.
- Mann JI, Vessey MP, Thorogood M, Doll R: Myocardial infarction in young women with special reference to oral contraceptive practice.
- Inman WHW, Vessey MP, Westerholm B, Engelund A: Thromboembolic disease and the steroidal content of oral contraceptives.
- Stolley PD, Tonascia JA, Tockman MS, et al: Thrombosis with low-estrogen oral contraceptives.
- Vessey MP, Doll R, Fairbairn AS, Glober G: Postoperative thromboembolism and the use of oral contraceptives.
- Greene GR, Sartwell PE: Oral contraceptive use in patients with thromboembolism following surgery, trauma or infection.
- Rosenberg L, Armstrong B, Phil D, Jick H: Myocardial infarction and estrogen therapy in post-menopausal women.
- Coronary Drug Project Research Group: The Coronary Drug Project: Initial findings leading to modifications of its research protocol.
- Baum J, Holtz F, Bookstein JJ, Klein EW: Possible association between benign hepatomas and oral contraceptives.
- Mays ET, Christopherson WM, Mahr MM, Williams HC: Hepatic changes in young women ingesting contraceptive steroids. Hepatic hemorrhage and primary hepatic tumors.
- Edmondson HA, Henderson B, Benton B: Liver-cell adenomas associated with use of oral contraceptives.
- Pfeffer RI, VanDenNoort S: Estrogen use and stroke risk in post-menopausal women.
12PATIENT INFORMATION
DEPO-Estradiol
Brand of estradiol cypionate injection, USP
Read this PATIENT INFORMATION before you start taking DEPO-Estradiol and read what you get each time you refill DEPO-Estradiol. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
12.1WHAT IS THE MOST IMPORTANT INFORMATION I SHOULD KNOW ABOUT DEPO-ESTRADIOL (AN ESTROGEN HORMONE)?
Estrogens increase the chances of getting cancer of the uterus.
Report any unusual vaginal bleeding right away while you are taking estrogens. Vaginal bleeding after menopause may be a warning sign of cancer of the uterine (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause.
Do not use estrogens with or without progestins to prevent heart disease, heart attacks, or strokes.
Using estrogens with or without progestins may increase your chances of getting heart attacks, strokes, breast cancer, and blood clots. You and your healthcare provider should talk regularly about whether you still need treatment with DEPO-Estradiol.
13PRINCIPAL DISPLAY PANEL - 5 mg/mL Vial Label
5 mL Vial
NDC 0009-0271-01
NDC 0009-0271-01
Depo
estradiol cypionate injection, USP
estradiol cypionate injection, USP
5 mg/mL
For intramuscular use only
Rx only

14PRINCIPAL DISPLAY PANEL - 5 mg/mL Vial Carton
NDC 0009-0271-01
1–5 mL Vial
Depo
estradiol cypionate
injection, USP
estradiol cypionate
injection, USP
5 mg/mL
For intramuscular use only
Pfizer Injectables